w w w . j c o l . o r g . b r
Journal
of
Coloproctology
Original
Article
Evaluation
of
the
anti-inflammatory
and
antioxidant
effects
of
the
sucralfate
in
diversion
colitis
夽
Carlos
Augusto
Real
Martinez
a,b,∗,
Murilo
Rocha
Rodrigues
c,
Daniela
Tiemi
Sato
c,
Camila
Morais
Gonc¸alves
da
Silva
d,
Danilo
Toshio
Kanno
e,
Roberta
Laís
dos
Santos
Mendonc¸a
e,
José
Aires
Pereira
caPost-GraduatePrograminHealthSciences,UnilversidadeSãoFrancisco(USF),Braganc¸aPaulista,SP,Brazil
bDivisionofColorectalSurgery,MedicalSciencesFaculty,UniversidadeEstadualdeCampinas(UNICAMP),Campinas,SP,Brazil cMedicineCourse,UniversidadeSãoFrancisco(USF),Braganc¸aPaulista,SP,Brazil
dGraduatePrograminFunctionalandMolecularBiology,UniversidadeEstadualdeCampinas(UNICAMP),Campinas,SP,Brazil
eResidentPhysician,ServiceofColoproctology,HospitalUniversitárioSãoFrancisconaProvidênciadeDeus,Braganc¸aPaulista,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received8December2014
Accepted20February2015
Availableonline29April2015
Keywords: Sucralfate Myeloperoxidase Malondialdehyde
Lipidperoxidation
Oxidativestress
Short-chainfattyacids
Rats
a
b
s
t
r
a
c
t
Sucralfateenemaspresentgoodresultsinthetreatmentofcolitis,howeverthemechanism
ofactionofthedrugisnotyetfullyclarified.
Objective:Toevaluatetheanti-inflammatoryandantioxidanteffectsofsucralfateenemas
indiversioncolitismodel.
Method:Thirty-six Wistar rats underwent intestinal bypass by end colostomy in the
descendingcolonanddistalmucousfistula.Theanimalsweredividedinto3
experimen-talgroupsaccordingtothedailydoseofenemasreceivedcontaining0.9%SF,sucralfate
enemasorsucralfateenemas1g/kg/dayor 2g/kg/day.Eachgroupwasdividedintotwo
subgroupsaccordingtoeuthanasiatobeperformed2–4weeksafterderivation.Thetissue
gradeofinflammationwasassessedhistologically,andneutrophilinfiltrationbythetissue
expressionofmyeloperoxidase(MPO)identifiedbyimmunohistochemistryandquantified
bycomputerizedmorphometry.Oxidativestresswasmeasuredbytissuelevelsof
malondi-aldehyde(MDA).TocomparetheresultstheStudent’sttestvariancewasused,andalsothe
variancebyANOVAtest,establishingalevelofsignificanceof5%(p<0.05)forboth.
Results:Theinterventionwithsucralfateenemasshowedimprovementintheintensityof
tissueinflammationrelatedtotheconcentrationusedandthedurationofthe
interven-tion.InterventionwithsucralfateenemasreducedthetissuelevelsofMPO,independent
ofconcentrationortimeofintervention(p<0.01).TherewasareductionofMDAlevelsin
animalsirrigatedwithsucralfateenemas,independentofconcentrationordurationofthe
intervention(p<0.01).
夽
StudyconductedattheMedicalResearchLaboratory,GraduatePrograminHealthSciences,UniversidadeSãoFrancisco,Braganc¸a
Paulista,SP,Brazil.StudyawardedwiththeprizeJournalofColoproctologyduringthe63rd.BrazilianCongressofColoproctologyCongress
(Brasilia(DF),2014).
∗ Correspondingauthor.
E-mail:carmartinez@uol.com.br(C.A.R.Martinez).
http://dx.doi.org/10.1016/j.jcol.2015.02.007
Conclusion: Enemaswithsucralfateenemasreduceinflammation,neutrophilinfiltration
andoxidativestressintheexcludedcolonsuggestingtopicalapplicationofthesubstance
tobeavalidtherapeuticoptionforthetreatmentofdiversioncolitis.
©2015SociedadeBrasileiradeColoproctologia.PublishedbyElsevierEditoraLtda.All
rightsreserved.
Avaliac¸ão
dos
efeitos
anti-inflamatório
e
antioxidante
do
sucralfato
na
colite
de
exclusão
Palavras-chave: Sucralfato Mieloperoxidase Malondialdeído
Peroxidac¸ãodoslípidos
EstresseOxidativo
Ácidosgraxosdecadeiacurta
(AGCC) Ratos
r
e
s
u
m
e
n
Aaplicac¸ãodeclisterescomsucralfato(SCF)apresentabonsresultadosnotratamentode
colites,entretantoseumecanismodeac¸ãoaindanãoencontra-seesclarecido.
Objetivo: Avaliarosefeitosanti-inflamatórioseantioxidantesdoSCFemmodelodecolite
deexclusão.
Método:Trintaeseisratos,foramsubmetidosaderivac¸ãointestinalporcolostomiaterminal
nocólondescendenteefistulamucosadistal.Osanimaisforamdivididosem3grupos
exper-imentaissegundoreceberemclisteresdiárioscomSF0,9%,SCF1g/kg/diaouSCF2g/kg/dia.
Cadagrupofoidivididoemdoissubgrupossegundoaeutanásiaserrealizadaapós2ou4
semanasdaderivac¸ão.Ograudeinflamac¸ãotecidualfoiavaliadoporestudohistológico
eainfiltrac¸ãoneutrofílicapelaexpressãotecidualdemieloperoxidase(MPO)identificada
porimunoistoquímicaequantificadapormorfometriacomputadorizada.Oestresse
oxida-tivofoimensuradopeloconteúdodemalondialdeído(MDA).Paraanálisedosresultados
utilizou-seostestetdeStudent,eANOVA,estabelecendo-separatodosostestesnívelde
significânciade5%(p<0,05).
Resultados: Aintervenc¸ãocomSCFmelhorouograudeinflamac¸ãotecidual
relacionando-seaconcentrac¸ãoutilizadaeaotempodeintervenc¸ão.Aintervenc¸ãocomSCFreduziu
osníveisteciduaisdeMPO,independentedaconcentrac¸ãooudotempodeintervenc¸ão
(p<0,01).Houvereduc¸ãodosníveisdeMDAnosanimaisirrigadoscomSCF,independente
daconcentrac¸ãooutempodeintervenc¸ão(p<0,01).
Conclusão: Enemas comSCFreduzemoprocesso inflamatório,infiltradoneutrofílico e
estresseoxidativonocólonexclusosugerindoqueasubstânciapossasetornarumaopc¸ão
terapêuticaválidaparaotratamentodacolitedeexclusão.
©2015SociedadeBrasileiradeColoproctologia.PublicadoporElsevierEditoraLtda.
Todoslosderechosreservados.
Introduction
Sucralfate(SCF)isformedbytheassociationbetweensucrose
octosulphateandpolyaluminumhydroxide.1 Formorethan
threedecadesSCFhasbeenusedasacytoprotective agent
fortreatmentofgastrointestinalulcerdiseases.2Studieshave
shownthatthetherapeuticeffectsofSCFappeartoberelated
toitsabilitytoadheretoerosionsandulcerationsin
gastroin-testinal mucosa, forming a difficult-to-remove mechanical
barrier.2 However,it was demonstrated later that SCF also
presents other mechanisms of action.3,4 This drug
stimu-latesthesecretionofprostaglandinE2(PGE2),thusincreasing
theproductionandsecretionofmucusbygobletcellsofthe
gastrointestinalepithelium.2SCFenhancestheproductionof
epidermalgrowthfactor(EGF),whichinducescelldivisionand
promotes tissuereepithelialization.3 SCF has antimicrobial
activity,actingagainstthepathogenicbacterialflorapresent
inthecoloniclumen.3IthasbeenshownalsothattheSCF
moleculehasremarkableantioxidant activity,reducing the
productionandremovingoxygenfreeradicals(OFR)presentin
inflamedtissues.4Thisantioxidantactionprotectsthe
epithe-lial cellsofgastrointestinalmucosaagainstperoxidationof
phospholipids,the mainconstituentsofcytoplasmic
mem-branes, thus reducing apoptosis.3–5 All of these properties
haveledseveralauthorstouseSCFfortreatmentofcolorectal
inflammatorydiseases.2,6–11Theresultsofthesestudies
con-firmthattheuseofSCFenemaswaseffectiveinhealingulcers
ofrectalmucosa,asthosefound,forexample,inactinic
rec-titis,ulcerativecolitis,solitaryulceroftherectumand,more
recently,diversioncolitis(DC).2,6–11
DC is an inflammatorybowel disease (IBD) that has its
onsetincolonsegmentsexcludedfromintestinaltransit.12It
hasbeenshownthatepithelialcellsoftransit-excluded
seg-ments,devoidoftheirprimaryenergysupply,representedby
short-chainfattyacids(SCFA),undergochangesintheir
res-piratorymetabolism–increasing,asaresult,theformation
ofOFR.13,14Theresultingoxidativestresscausesbreakdown
ofthosevariousdefensesystemsthatformtheepithelial
pro-tectivebarrier.15–17Theruptureofthesedefensemechanisms
enables the invasionofsterilelayers ofthe intestinalwall
response.13,14Inthisprocess,theintenseneutrophilmigration
furtherincreasesOFRproduction,perpetuatingtheepithelial
aggressionthatcharacterizesDC.13
When one considers that the oxidative stress resulting
fromanincreasedtissueproductionofOFRbymucouscells
devoidoffecaltransit,aswellasneutrophilinfiltration,are
mechanisms involvedin DC etiopathogenesis, it willbe of
interest to evaluate the antioxidant activity of SCF in an
experimentalmodelofDC.IfSCFisabletoenhancethe
inflam-matoryprocessatmucosallevel,reduceneutrophilinfiltration
anddiminishOFRproduction,thisdrugcouldbecomea
valu-ablealternative fortreatmentofDC.Theaimofthis study
wastoevaluateantioxidantandanti-inflammatoryeffectsof
atopicalapplicationofSCFinanexperimentalmodelofDC.
Method
ThisstudyfollowedtherecommendationsoftheFederalLaw
11,794and oftheBrazilianCollegeofAnimal
Experimenta-tion(COBEA).TheresearchprojectwasapprovedbytheEthics
CommitteeonAnimalUseinResearch(CEUA),Universidade
deSãoFrancisco(OpinionN◦22-11/2007).
Experimentalanimals
Forthepresentstudy,36maleWistarratsweighing300–350g,
providedbytheCentralAnimalFacility,UniversidadedeSão
Francisco, were used. Theanimals were kept inindividual
cagesundercontrolledconditionsoftemperature,humidity,
lightandnoise.Priortothesurgicalprocedure, allanimals
werefasted,butwithwater,for12h.Thecageswere
identi-fiedwiththenumberoftheratandtheexperimentalgroupto
whichitbelonged.Thesesamedataweretattooedonthetail
ofeachrat.
Surgicaltechnique
Thederivationoftheintestinaltransitwasperformedinall
animalsundergeneralanesthesia.Onthedayofsurgery,the
animals were weighed to calculate the anesthetic dose. A
1:1solution containing xylazine 2%(Anasedan, Agribrands
doBrasilLtda., São Paulo,Brazil) and ketamine
hydrochlo-ride(Dopalen,AgribrandsdoBrasilLtda.,SãoPaulo,Brazil.)
inadoseof0.1mL/100gintramuscularlyinthelefthindpaw
wasusedasanestheticvehicle.Onceanesthetized,the
ani-malswerefixedontheoperatingtableandtheentireanterior
abdomenwas shaved.Then antisepsiswith PVP(povidone
iodine,10%topicalsolution)wascarriedout.Theabdominal
cavitywasaccessedviaa3-cmlengthmidlineincision.After
examinationofthecavity,thePeyer’spatch,alymphoid
struc-turelocatedontheanteriorfaceofthecolon,inthetransition
betweentheanimal’srectumandsigmoidcolon,was
identi-fied.Withtheaidofacaliper,theleftcolonwassectionedat
astandarddistance4cmabovetheupperlimitofthePeyer’s
patch.Thecolon’sproximalsegmentwasexternalizedinthe
formofaterminalcolostomyontheleftflank,andfixedto
theskinwithseparatepointsof4.0monofilamentabsorbable
suture applied in four cardinal points and between these
points.Afterproximalstomamaturation,thecaudalsegment
36 animals
Control (n=12)
SCF (1.0 g/kg/day) (n=12)
SCF (2.0 g/kg/day) (n=12)
Euthanasia 2 weeks
(n=6)
Euthanasia 4 weeks
(n=6)
Euthanasia 2 weeks
(n=6)
Euthanasia 4 weeks
(n=6)
Euthanasia 4 weeks
(n=6) Euthanasia
2 weeks (n=6)
Fig.1–Distributionofexperimentalgroups.
ofthesectionedcolonwascatheterizedwitha12F
polyethy-lenetubeandsubjectedtoirrigationwith40mLof0.9%saline
solutionat37◦Cuntilthewholeeffluentdrainedbythe
ani-mal’sanus(previouslydilated)nolongerdisplayedanyfecal
waste output.Afterthis mechanicalcleaning, the catheter
was removedand thedistalcolonexteriorized throughthe
abdominalwallasamucousfistulainthelowerleftfaceof
theabdominalwall.Thecaudalcolostomy wasfixedtothe
skinwiththesametechniquedescribedforthecranialstoma.
Afterstomafixation,theabdominalwallwasclosed intwo
surgicalplans:peritoneumandaponeurosiswith3–0braided
polyglycolic acid suture,and the skinwith separatepoints
of4–0monofilamentnylon.Postoperatively,theanimalsdid
notreceiveantibiotics,andnofurthercareregardingsurgical
incisionandthestomatawastaken.
Experimentalgroups
Fig.1showsthedistributionofourexperimentalgroups.Our
36 animals were divided randomlyinto threegroups of12
ratseach.Inthefirstgroup,theanimalsweresubjectedtoan
applicationofenemaswith0.9%salinewarmedtoroom
tem-perature(controlgroup).Thesecondandthethirdgroupsof
animals(experimentalgroups)receiveddailyapplicationsof
enemascontainingSCF(EMSdoBrasilLtda.,SãoPaulo,Brazil)
intwodifferentconcentrations(1.0g/kg/dayand2.0g/kg/day,
respectively). Inall animals the applicationofintervention
solutionswascarriedoutwiththeaidofaninfusionpump
(KDScientificInc.,Holliston,MA,USA)atacontrolledinfusion
rateof20mL/min.Ineachofthethreeexperimentalgroups,
sixanimalsweresacrificedaftertwoweeks,andtheothersix
afterfourweeks.
Samplecollection
In thescheduleddatesforeuthanasia,animals were
anes-thetized with the same technique described above. The
abdomen was reopened through a midline incision with
greaterlength.Theexcludedcolon(subjectedtothe
interven-tionsolutions),includingtheanusofallanimals,wascarefully
removed.Theremovedcolonsegmentswereopenedthrough
their anti-mesentericborder and rinsed with PBS solution
heated at37◦C for 2min. A fragment with 3cm inlength
(excludingtheanus andtheregionclosesttothestoma)of
eachanimalwasfixedwiththemucosafacinguponapiece
theaidofpins.Afterthisfasteningprocedure,thematerial
wasplacedintovialscontaining10%bufferedformaldehyde
(Sigma,St.Louis,MO,USA).Duringremovaloftheexcluded
colon,asecondfragmentmeasuring1cmwasalsoremoved,
washedwithPBSsolutionat37◦C,packagedincryoflasksand
storedunderultra-coolingconditions,forfurtherbiochemical
analysisoftissuelevelsofmalondialdehyde(MDA).
Histologicalanalysis
Thefragmentsdesignatedforhistologicalstudywerekeptin
10% formaldehyde for48h atroom temperature toensure
properspecimenfixation.Then, thespecimenswere
dehy-dratedbyexposuretoincreasingconcentrationsofethanol
andembeddedinparaffin.Fromeachblock,two5m-thick
fragmentswerecutwiththeaidofamanualmicrotome(Leica
RM2235,Leica doBrasilImportac¸ão eComércio Ltda.,São
Paulo,Brazil),forslidemounting.Oneslidewasstained by
hematoxylin–eosin(HE)techniqueand sentfor
histopatho-logicalevaluationforthepresenceofcolitis,aswellasforthe
degreeoftissueinflammation.Thesecondslidewasintended
forimmunohistochemistry, to detect the tissue expression
of myeloperoxidase (MPO). All slides were analyzed with
an ordinary optical microscope (Eclipse DS-50, Nikon Inc.,
Osaka,Japan)byapathologistspecializedinIBDdiagnosisand
blindedfortheoriginofthematerialandthestudyobjectives.
Histologicalphotographsweretakenusingadigitalvideo
cam-era(DS-Fi-50, NikonInc.,Osaka, Japan)previouslyattached
tothemicroscope body.Allspecimensanalyzedwere
pho-tographedwith a final magnification of100×. Thereading
ofeach slidewasalwaysdone inahistologicalfield
show-ingatleastthreeintactandcontiguous colonicglands.For
each slide,threedistinct histologicalfields were evaluated.
The diagnosis of colitis and the degree of tissue
inflam-mation were determined by histological (modified)criteria
previouslydescribedbyAkgunetal.18(Table1).Thefollowing
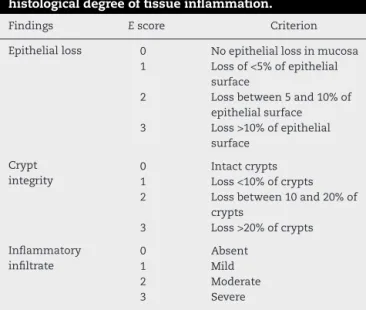
Table1–Variablesusedforstratificationofthe histologicaldegreeoftissueinflammation.
Findings Escore Criterion
Epithelialloss 0 Noepitheliallossinmucosa 1 Lossof<5%ofepithelial
surface
2 Lossbetween5and10%of epithelialsurface 3 Loss>10%ofepithelial
surface
Crypt integrity
0 Intactcrypts
1 Loss<10%ofcrypts 2 Lossbetween10and20%of
crypts
3 Loss>20%ofcrypts
Inflammatory infiltrate
0 Absent
1 Mild
2 Moderate
3 Severe
ModifiedfromAkgunetal.18
stratification forhistologicaldegree oftissueinflammation
wasadopted:0–3,mild;4–6,moderate;and7–9,severe.
ImmunohistochemistryforstudyoftissuelevelsofMPO
Fortheimmunohistochemicalstudy,allblocksweresectioned
in5m-thicksectionsobtainedfromcolonsegmentstreated
withtheinterventionsolutions.Thesecutsweredepositedin
previouslysilanizedslidesandidentifiedwiththenumberof
theratandthegrouptowhichhebelonged.Slideswere
diaph-anizedandrehydrated,andantigenretrievalwasperformed
usingtheTrilogysolution(CellMarkInc.,Rocklin,CA,USA).
Next,theslideswererinsedwithdistilledwaterand
subse-quentlyimmersedinPBSsolutionfor10minanddriedwith
filterpaper.Endogenousperoxidaseswereblockedusing3%
hydrogenperoxide(H2O2)inahumidchamberatroom
tem-perature for10min. Then, furtherwashing was performed
withPBSfor10min.Afterthisprocess,theslideswereleft
rest-ingatroomtemperaturefor10minandthenwashedwithPBS
for5min.Theprimarypolyclonalanti-MPOantibody(Dako
doBrazilLtda.,SãoPaulo,Brazil)withcross-reactivitytorats
wasdilutedinsalinecontainingbovineserumalbumin(1%)
diluted1:100.Allslideswerecoatedwith100Lofthissolution
andleftrestingatroomtemperatureforaperiodof2h.
Follow-ingexposuretoprimaryantibody,theslideswererinsedwith
distilledwater(2baths)andPBSbuffer(twobathsof2min).
Then,theslideswereincubatedwithanavidin–biotinsystem
(secondaryantibody) comprisingtheLSAB+kit System-HRP
(DakodoBrasilLtda.,SaoPaulo,Brazil)fora35-minperiodof
exposureforeachreagent,andthenwashedwithtwobaths
ofPBS.ThesectionprocessingoccurredbyusingtheLiquid
DAB+SubstrateKit(DakodoBrasilLtda.,SãoPaulo,Brazil)in
adilutionof1dropofchromogenoussolutionin1Lofbuffer
solution. 100Lofthechromogen wasaddedover the
sec-tions foraperiodof5minatroomtemperature. Afterthis
processing,thesectionswerewashedinrunningwaterand
counterstained withHarris hematoxylinfor30s. After this
process,theslideswereagainwashedinrunningwater,until
removalofexcessdye.Finally,theslidesweredehydratedin
threebathswithincreasingconcentrationsofalcoholandtwo
bathsofxylene.Theslideswerethenmountedwithcoverslips
andresin.
Computerizedmorphometry
The immunostaining was considered positive when a
dif-fusely brownish color was present, with variable intensity
points and a homogeneous distribution in neutrophils. As
recommendedbythemanufacturer,thenegativecontrolfor
the immunostaining was performed without the addition
ofprimaryantibody; andthe positivecontrolwas madein
humanvermiformappendixsufferingfromacute
appendici-tis. Thepresenceofa brownish color makesit possibleto
quantify its tissue content by computerized morphometry
(computer-assistedimageprocessing).TheMPOcontentwas
then measured with the aid of NIS-Elements 4.0 software
(NikonInc.,Osaka,Japan).Likethehistologicalanalysis,the
tissuecontentofMPOineachsamplewasalwaysdetermined
inasitewheretherewereatleastthreecontiguouscrypts.
(Red,Green,Blue)systemisabletodeterminetheamountof
theselectedcolor(inthiscasethebrowncolor,thatinwhich
MPOisexpressed)presentinthetissue,convertingcolor
inten-sityinto number ofpixelsineachselected field.Thus,the
finalcontentofMPOwasdeterminedinpercentagebyfield
(%/field).Thefinalamountconsideredforeachratrepresented
the average valueobtained afterreading three histological
fieldsintheestablishedmagnification(100×).
Determinationofmalondialdehyde(MDA)levels
Thelevelsoflipidperoxidationwereevaluatedbymeasuring
thelevelsofthiobarbituricacidreactivesubstances(TBARS),
aswithMDA,withapreviouslydescribedmethodology.19MDA
isasecondaryproductoflipidoxidationandisconsidereda
potentialcandidateforbeingageneralbiomarkerof
oxida-tivestress.AstothequantificationoftissuelevelsofMDA,
1gofeachfragmentwasplacedin5mLofphosphatebuffer
andhomogenizedbyvortexandultrasoundsonicationfor30s,
alternately,repeatingtheprocessforthreetimes.Then,250L
ofthesupernatantobtainedfromthehomogenizationprocess
wastransferredtoaplastictesttubecontaining25mLof4%
methanolicBHT,withanewvortexhomogenization.The
sam-plewasthenmixedwith1mLof12%trichloroaceticacid,1mL
of0.73%thiobarbituricacidand750LofTris/HClbuffer,and
thenincubatedinawaterbathat100◦Cfor60min.Afterthis
step,thetubeswereimmediatelyplacedinacontainerwith
icetoblockthereaction,withadditionof1.5mLofn-butanol.
Then, the mixture was again vortexed for 30s. The
sam-pleswereseparatedbycentrifugationfor10minat5000rpm.
Finally,thesupernatantwasremoved,andtheabsorbanceat
532nmoftheorganicphasewasanalyzed,usingaUV/vis6105
(Jenway,BibbyScientificLimited, Cheshire,UK)
spectropho-tometer.
Statistical
analysis
Theresultsforthe degree ofinflammationwere described
accordingtothemedianofthevaluesobtained.Astotissue
levelsofMPOandMDA,theresultsweredescribedaccordingto
theirmedia±standarderror.Thecomparisonofresultsfound
amongexperimentalgroupswasanalyzedbyStudent’sttest.
ANOVAtestwasusedtostudythevariationinresults
accord-ingtotheinterventiontimeineachexperimentalgroup.Itwas
establishedforallteststhelevelofsignificanceof5%(p<0.05),
andweusedoneasterisk(*)toidentifyvaluesofp<0.05and
twoasterisks(**)forvaluesofp<0.01.
Results
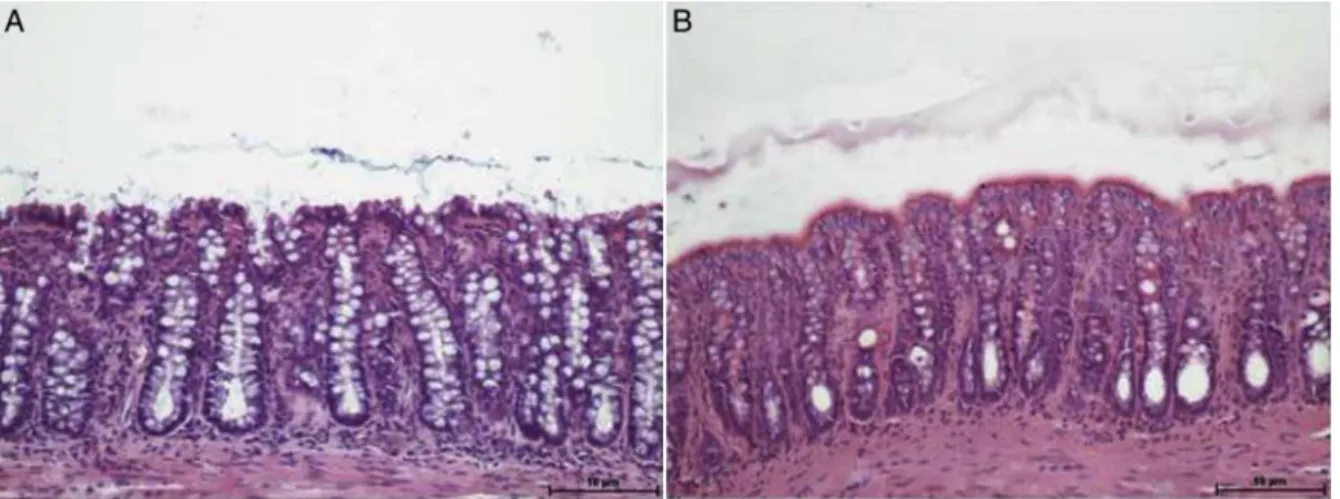
Fig.2Ashowscolonicepitheliumexcludedfrombowel
tran-sit,submittedtointerventionwith0.9%salinefor4weeks,
while Fig.2Bshowsthecolonwithouttransitsubmittedto
irrigationwithSCFataconcentrationof2.0g/kg/day.
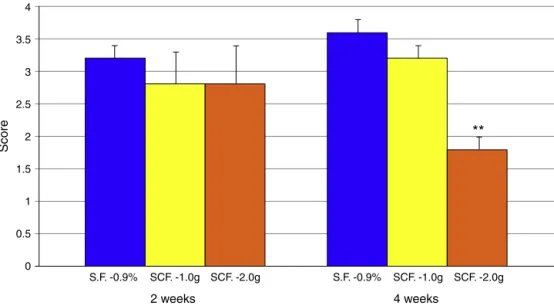
Fig.3showsthedegreeoftissueinflammation,compared
withanimalssubjectedtointerventionwith0.9%saline,SCF
1.0g/kg/dayandSCF2.0g/kg/dayfor2and4weeks.
Fig.4AshowsthetissueexpressionofMPOinthesegments
withouttransitandsubjectedtointerventionwith0.9%saline
for4weeks,whileFig.3Bshowsthecolonwithouttransit
sub-jectedtoirrigationwithSCFataconcentrationof2.0g/kg/day
for4weeks.
Fig.5showsMPOtissuecontentbycomparinganimals
sub-jectedtointerventionwith0.9%saline,SCF1.0/kg/dayandSCF
2.0/kg/dayfor2–4weeks.
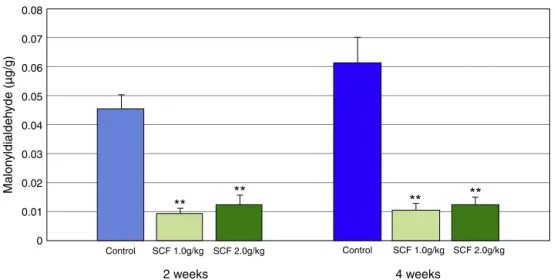
Fig.6showsMDAtissuecontentbycomparinganimals
sub-jectedtointerventionwith0.9%saline,SCF1.0g/kg/dayand
SCF2.0g/kg/dayfor2and4weeks.
Discussion
Thecolonicepitheliumisconsideredthemostperfect
func-tionalbarrierofthehumanbody.13Formedbyasinglelayerof
cells,itseparatesthecoloniclumen,anantigenand
bacteria-richenvironment,fromthesterileinternalenvironment.16,17
This property becomes important when we consider that
in the early stages ofIBD, especially ulcerativecolitis, the
colonicmucosaisconsistentlyimpaired,suggestingthatthe
4
3
2
1 3.5
2.5
1.5
0.5
S.F. -0.9% SCF. -1.0g SCF. -2.0g S.F. -0.9% SCF. -1.0g
Score
SCF. -2.0g
**
0
2 weeks 4 weeks
Fig.3–Degreeofinflammationcomparinganimalssubjectedtointerventionwith0.9%saline,SCF1.0g/kg/dayand 2.0g/kg/dayfor2and4weeks.**Significant:(SCF2.0g/kg/day×0.9%salineandSCF1.0g/kg/day)(p<0.01).Student’sttest.
disruptionofthe epithelialbarrierisanearlyeventrelated
to the pathogenesis of the disease.14 The importance of
epithelialbarrierintegrityisenhancedwhenoneconsiders
that the major experimental models proposed for colitis
inductionuse chemicalssuchas trinitrobenzenesulphonic
acid(TNBS), H2O2,acetic acid and dextransulfateinorder
todisruptthe epithelialbarrier,thus allowingthebacterial
invasionandconsequentlyanacuteinflammatoryresponse
thatcharacterizesthedisease.20
However,theearlymolecularmechanismsthattriggerthe
injuryofthosedifferentdefensesystemsthatformthecolonic
barrierinpatientswithIBDhavenot beenfully clarified.14
Oneofthepossiblepathogenicmechanismsinvolvedinthe
initialinjuryofthecolonicmucosalbarrierwassuggestedby
Pravdain2005,thatproposedthetheoryofcolitisinductionby
OFR.14AccordingtoPravda,thepathogenesisofcolitishadtwo
distinctstages.Inthefirststage,calledbytheauthoras
“ini-tiationphase”,theinitialinsulttotheintestinalmucosawas
aresultoftheincreasedproductionofOFRbytheepithelial
cellsofthecolonmucosathemselves,withchangesintheir
energymetabolism.TheoverproductionofOFRbythesecells
would cause breakageofthose differentdefenselines that
makeupthemucosalbarrier,allowingmigrationofbacteria
and antigenspresentinthe intestinallumeninto the
ster-ileintimacyofthesubmucosa.14Inthesecondstage,called
“spreadingphase”,neutrophilswouldmigrateintothe
intesti-nalwall inanattempt tocombatthe bacterial infiltration,
disseminatingtheinflammatoryprocess.14,21,22The
possibil-itythattheincreasedproductionofOFRmaycauseinjuryto
thecolonicmucosalepitheliumhasbeenknownforseveral
years,whenitwasshownthatH2O2(apotentOFRgenerator)
instillationinsidethecolonwasfollowedbyaseverepictureof
colitis,sometimeswithafataloutcome.23–25Itshouldbenoted
thatH2O2-inducedcolitispresentsclinical,macroscopicand
microscopicfeaturesverysimilartothosefoundinulcerative
colitis.26
16.00
12.00
10.00
8.00
6.00
4.00
.00 2.00 14.00
SCF 1.0g/kg SCF 2.0g/kg
2 weeks
**
**
**
**
Control Control SCF 1.0g/kg SCF 2.0g/kg
4 weeks
MPO activity (%/field)
Fig.5–MPOtissuecontent,comparinganimalssubjectedtointerventionwith0.9%saline,SCF1.0g/kg/dayandSCF 2.0g/kg/dayfortwoandfourweeks.**Significant:SCF2.0g/kg/dayandSCF1.0g/kg/day×0.9%saline(p<0.01).Student’st test.
However, in order to experimentally verify if the colon
mucosacellswithchangesintheirenergymetabolismwould
beabletoproduceOFRinsufficientquantitytodamagethe
intestinalepithelium,it wouldbenecessarytoestablish an
experimentalmodel of colitis where the initialdamage to
themucosawasnotcausedbythe exposureofthe
intesti-nalepitheliumtochemicals,butbyconditionsthatonlywould
alterthecellularmetabolism.13SCFA,representedbybutyrate,
propionateandacetate,accountfor90%ofallsubstrateused
bycolonicmucosacellstoobtainenergy,andthesimple
depri-vationofthesesubstancestothemucosaalterstheenergy
metabolism of colonocytes, leading to the appearance of
DC.2,13,16,17Inthefaceofthisevidence,anexperimentalmodel
ofDCwouldassesstheabilityofepithelialcellsexcludedfrom
boweltransitinproducing largeramounts ofOFR,andalso
wouldverify if theresulting oxidativestresscould damage
thedifferentlinesofepithelialdefense.Anumberofstudies
usinganexperimentalmodelofDCshowedthatthecolonic
mucosa cells excluded from fecal transit are subjected to
tissueoxidativestressthroughanincreaseintheproduction
ofOFR.13Ithasalsobeendemonstratedthatan
overproduc-tionofOFRcausesharmtothecolonicmucosa,decreasesthe
populationofgobletcells,reducesthecontentandmodifies
theexpressionofmucins,causesdisruptionofprotein
con-stituentsofintercellularjunctions,andalsocausesoxidative
damage tocellularDNA.13,15–17,27–29 All these findings
con-firmedthehighercapacityofOFRproductionbycoloniccells
withmetabolicchanges,asshownbytherelationshipbetween
anincreasedproductionofOFRandthedisruptionofdifferent
defensesystemsofepithelialbarrier.13
Basedonthesefindings,severalstudiesbegantotestthe
effectivenessofsubstanceswithantioxidantactivityforthe
treatmentofDC.19,30–32Theresultsofthesestudiesconfirmed
thatnaturalorsyntheticsubstanceswithantioxidantactivity
wereabletoreducetheproductionofOFRandtoimprovethe
histologicalchangesthatcharacterizeDC.19,29–32
Rileyetal.in198933werethefirsttodemonstratethe
ben-efitsofusingenemaswithSCFintheacutephaseofulcerative
0.08
0.06
0.05
0.04
0.03
0.02
0 0.01 0.07
SCF 1.0g/kg SCF 2.0g/kg
2 weeks
**
**
**
**
Control Control SCF 1.0g/kg SCF 2.0g/kg
4 weeks
Malon
yldialdeh
yde (µg/g)
rectitis.Subsequently,otherstudieshaveconfirmedthe
effi-cacyofthedruginotherformsofcolitis.5,6,8–10Mostofthese
authorsattributedtheactionofthedrugtoitsadhesive
capac-ityontheinflamedepithelium.Rileyetal.in198933werethe
firsttodemonstratethe benefitsofusingenemaswithSCF
intheacutephaseofulcerativeproctitis.Subsequently,other
studieshaveconfirmedtheefficacyofthedruginotherforms
ofcolitis.5,6,8–10 Mostoftheseauthors attributedthe action
ofthedrugtoitsadhesiveproprietyontheinflamed
epithe-lium,regardlessofitsimportantantioxidantaction,already
knownfordecades.7,34 Theantioxidant activityofSCFwas
theresultofthedrug’sabilitytoremovetheOFRformedin
thetissuesunderdifferentexperimentalconditions.35–37Only
one study evaluated the effects ofthe application of
ene-maswithSCFinanexperimentalmodelofDC.2Theauthors
foundthatthissubstancereducedsignificantlytheepithelial
loss,decreasedtheabscessformationwithinintestinalcrypts,
andalsoreducedtheinflammatoryinfiltrateandlocal
colla-gendeposition,2inlinewithotherstudiesthatattributedthe
improvementofthemucousinflammatoryprocesstotheSCF
abilitytoformaprotectivelayeron theinflamed mucosa.2
Whiledrawingattentiontoothermechanismsofactionofthis
drug,thesestudiesdidnotassessthepossibilitythatthe
ben-eficialeffectsofthesubstancecouldberelatedtoapossible
antioxidantaction.2
Inthisstudy,weaimedtoverifyifthetherapeuticaction
ofSCFcouldberelatedtoitsantioxidantproperties.Weused
theDCmodel,becauseoxidativestressisnowconsideredone
ofthemolecularmechanismsrelatedtothe
etiopathogene-sisofthisdisease.13–17WiththeDCmodel,wecouldconfirm
theresultspreviouslydescribed,asweshowedthattheuseof
enemaswithSCFreducedthedegreeoftissueinflammation.2
Using apreviouslyvalidated inflammatoryscale,we found
thatthedegreeoftissueinflammationdecreasedsignificantly
inanimalsinwhichweappliedenemaswithhigherSCF
con-centrationsforalongerperiod.Thesefindingsshowedthat
thetopicaleffectofSCFdependsontheconcentrationused
andtheinterventiontime.Inall animalswhichunderwent
interventionwithSCF,wecoulddetect,throughthe
histolog-icalstudy,theformationofathinprotectivefilmcoveringthe
colonmucosainmostanimals.Thisfinding,coupledwiththe
improvementofthedegreeoftissueinflammation,confirms
theabilityofthisdrug,inactingasamechanicalbarrier
hin-deringthecontactbetweentheepitheliumwiththeexisting
floraintheintestinallumen.However,iftheactionofthisdrug
waspurelymechanical,byforcethetissueinflammationscore
shouldbelowerfromthefirstweeksofintervention,which
suggeststhatothermechanismsofactionareinvolved.
Thecolonicmucosainfiltrationbyinflammatorycells is
another common finding in DC. In more severe cases, in
the acute phase the infiltrate is composed predominantly
ofneutrophils, whereas inthe chronicphase, the
lympho-cytesbecomethemaincells,althoughstillwithneutrophils
present intissues.15 MPOisan enzymefoundprimarilyin
theazurophilicgranulesofneutrophils.13WhileMPOmaybe
presentinotherinflammatorycells,itisestimatedthat95%of
allitscontentcomesfromtheneutrophils;thisfindingmakes
thissubstanceanefficientmarkerforthepresenceofanacute
inflammatoryinfiltrate.Previousstudiesusedtissuedosageof
MPOcontentinordertoconfirmthepresenceofaneutrophil
infiltrateinDC.13,38Inthisstudy,whenweanalyzethe
infiltra-tionofneutrophilsforassessingthetissuecontentofMPO,we
foundthatanimalssubjectedtoSCFinterventionhadalower
contentofMPOcomparedtoanimalsreceivingintervention
with0.9%saline.Thereductioninneutrophilicinfiltratewas
notrelatedtotheconcentrationofSCFusedortothe
inter-ventiontime.Thereareseveralpossibleexplanationsforthis
finding.Perhapstheformationofamechanicalbarrieroverthe
epithelialsurfacemadeitdifficultfortheoccurrenceofbowel
wallinvasionbybacteriafromthecoloniclumen,decreasing
theneutrophilinflammatoryresponse.Possiblythe
antimicro-bialpropertiesofSCFcoulddecreasethenumberofbacteriain
bowellumen,thusdecreasingtheneutrophilinfiltration.
Like-wise,ifSCFcanreduceOFRproduction,theepithelialinjury
willbesmaller,whichwoulddecreasethebacterialinfiltration.
Inturn,thepenetrationofalowernumberwoulddiminishthe
infiltrateand,thus,theproductionofOFRfromneutrophils,
whichwouldconfirmtheantioxidantactivityofSCF.
ToevaluatetheantioxidantactionofSCF,weusedMDA
tissuecontentdosage.Theproductsoflipidperoxidationof
phospholipidspresentincellmembranes,suchasMDA,can
beusedasindicatorsofOFRactioninthebody.39Byfar,MDA
determinationisthemostpopularindicatorofoxidative
dam-agetocellsandtissues.39Wenotedthatinthefirsttwoweeks
ofinterventionwithSCF,asignificantreductioninMDAtissue
contentalreadyhadtakenplace,evenwhenweappliedalower
concentrationofthesubstance.MDAtissuecontentremained
lowafterfourweeksofintervention,regardlessofSCF
con-centrationused.Thisshowedthatthesubstancemaintained
its antioxidant activity. On the other hand, in the control
groupanimals,MDAlevelsprogressivelyincreasedwiththe
passageoftime,showingthatthelongertheepithelialcells
weredeprivedoftheirSCFAsupply,thehigherthelevelof
tis-sueoxidativestress.Thesefindingsconfirmtheantioxidant
powerofSCF,sincetheactionofthisdrugisnotdependent
ontheconcentrationusedorontheinterventiontime.The
decreaseinMDAlevelswasdirectlyrelatedtothe
improve-mentoftheneutrophilicinfiltratewithinthe firstweeksof
intervention,remainingthatwaythroughouttheintervention
period.ThelowerMDAcontentwasalsodirectlyrelatedtothe
improvementofthedegreeofinflammationafterfourweeks
ofintervention.ThisfindingshowsthatSCFreducesoxidative
stressintissueandpreservestheepithelialbarrier,resulting
inasmalleracuteinflammatoryresponse.
TheresultsofthisstudyconfirmthatSCFhasantioxidant
action,as previouslyshown.5 When oneconsidersthat DC
pathogenesisisrelatedtooxidativestress,theresultssuggest
thattheapplicationofenemaswithSCFisanewandeffective
therapeuticapproachforthetreatmentofthisdisease.Itslow
cost,easyavailabilityandthelackofserioussideeffectsare
additionaladvantagestobeconsidered,tominimizethe
suf-feringofpatientswithDCalreadylivingwiththelimitations
imposedbythepresenceofastoma.
Conclusion
Undertheconditionsofthisexperimentalstudy,weconclude
thattheapplicationofenemascontainingSCFinacolonwith
decreasesneutrophilinfiltrationandimprovestissue
inflam-mation,confirmingtheantioxidantactionofthissubstance.
Funding
Fundac¸ão de Amparo à Pesquisa do Estado de São Paulo
(FAPESP).ProcessN◦2010/12492-7.
Conflict
of
interests
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. VolkinDB,VerticelliAM,MarfiaKE,BurkeCJ,MachH, MiddaughCR.Sucralfateandsolublesucroseoctasulfatebind andstabilizeacidicfibroblastgrowthfactor.BiochimBiophys Acta.1993;1203:18–26.
2. PereiraJA,RodriguesMR,SatoDT,etal.Evaluationofthe effectsofsucralfateenemainexperimentaldiversioncolitis.J Coloproctol(RioJ).2013;33:182–90.
3. WestAP,AbdulS,SherrattMJ,InglisTJ.Antibacterialactivity ofsucralfateagainstEscherichiacoli,Staphylococcusaureusand Pseudomonasaeruginosainbatchandcontinuousculture.EurJ ClinMicrobiolInfectDis.1993;12:869–71.
4. WadaK,KamisakiY,KitanoM,KishimotoY,NakamotoK, ItohT.Effectsofsucralfateonacutegastricmucosalinjury andgastriculcerinducedbyischemia–reperfusioninrats. Pharmacology.1997;54:57–63.
5. KochharR,MehtaSK,AggarwalR,DharA,PatelF.Sucralfate enemainulcerativerectosigmoidlesions.DisColonRectum. 1990;33:49–51.
6. WrightJP,WinterTA,CandyS,MarksIS.Sucralfateand methylprednisoloneenemasinactiveulcerativecolitis:a prospective,single-blindstudy.DigDisSci.1999;44:1899–901. 7. Matsuu-MatsuyamaM,ShichijoK,OkaichiK,etal.Sucralfate
protectsintestinalepithelialcellsfromradiationinduced apoptosisinrats.JRadiatRes.2006;47:1–8.
8. DentonAS,AndreyevHJN,ForbesA,MaherEJ.Systematic reviewfornon-surgicalinterventionsforthemanagementof lateradiationproctitis.BrJCancer.2002;87:134–43.
9. HensonC.Chronicradiationproctitis:issuessurrounding delayedboweldysfunctionpost-pelvicradiotherapyandan updateonmedicaltreatment.TherAdvGastroenterol. 2010;3:359–65.
10.MendenhallWM,McKibbenBT,HoppeBS,NicholsRC, HendersonRH,MendenhallNP.Managementofradiation proctitis.AmJClinOncol.2013;March[Epubaheadofprint]. 11.DehghaniSM,MalekpourA,HaghighatM.Solitaryrectalulcer
syndromeinchildren:aliteraturereview.WorldJ Gastroenterol.2012;18:6541–5.
12.GlotzerDJ,GlickME,GoldmanH.Proctitisfollowingdiversion offecalstream.Gastroenterology.1981;80:438–41.
13.MartinezCAR,RibeiroML,GamberoA,MirandaDDC,Pereira JA,NadalSR.Theimportanceofoxygenfreeradicalsinthe etiopathogenesisofdiversioncolitisinrats.ActaCirBras. 2010;25:387–95.
14.PravdaJ.Radicalinductiontheoryofulcerativecolitis.WorldJ Gastroenterol.2005;11:2371–84.
15.SousaMV,PriolliDG,PortesAV,CardinalliIA,PereiraJA, MartinezCA.Evaluationbycomputerizedmorphometryof histopathologicalalterationsofthecolonwallinsegments withandwithoutintestinaltransitinrats.ActaCirBras. 2008;23:417–24.
16.MartinezCAR,NonoseR,SpadariAP,etal.Quantificationby computerizedmorphometryoftissuelevelsofsulfomucins andsialomucinsindiversioncolitisinrats.ActaCirBras. 2010;25:231–40.
17.NonoseR,SpadariAPP,PriolliDG,MaximoFR,PereiraJA, MartinezCAR.Tissuequantificationofneutralandacid mucinsinthemucosaofthecolonwithandwithoutfecal stream:experimentalstudyinrats.ActaCirBras. 2009;24:267–75.
18.AkgunE,C¸aliskanC,CelikHA,OzutemizAO,TuncyurekM, AydinHH.EffectsofN-acetylcysteinetreatmentonoxidative stressinaceticacid-inducedexperimentalcolitisinrats.JInt MedRes.2005;33:196–206.
19.MartinezCA,deAlmeidaMG,daSilvaCM,etal.Enemaswith N-acetylcysteinecanreducethelevelofoxidativedamagein cellsofthecolonicmucosadivertedfromthefaecalstream. DigDisSci.2013;58:3452–9.
20.GoyalN,RanaA,AhlawatA,BijjemKR,KumarP.Animal modelsofinflammatoryboweldisease:areview. Inflammopharmacology.2014;22:219–33.
21.MillarAD,RamptonDS,ChanderCL,etal.Evaluatingthe antioxidantpotentialofnewtreatmentsforinflammatory boweldiseaseusingaratmodelofcolitis.Gut.1996;39: 407–15.
22.CadenasE,DaviesKJ.Mitochondrialfreeradicalgeneration, oxidativestress,andaging.FreeRadicBiolMed.
2000;29:222–30.
23.SheehanJF,BrynjolfssonG.Ulcerativecolitisfollowing hydrogenperoxideenema:casereportandexperimental productionwithtransientemphysemaofcolonicwalland gasembolism.LabInvest.1960;9:150–68.
24.AlmaloufP,ShehabTM,DanielAM,RobinsonEA,BarnettJL. Therapeutichydrogenperoxideenemacausingsevereacute colitis.IntJColorectalDis.2008;23:
1139–40.
25.KeshavarzianA,MorganG,SedghiS,GordonJH,DoriaM.Role ofreactiveoxygenmetabolitesinexperimentalcolitis.Gut. 1990;31:786–90.
26.MarquesLHS,SilvaCMG,LameiroTMM,etal.Avaliac¸ãodos níveisdeperoxidac¸ãolipídicaemcélulasdamucosacólica apósaplicac¸ãodeenemascomperóxidodehidrogênio: estudoexperimentalemratos.VerBrasColo-proctol. 2010;30:272–80.
27.MelloRO,SilvaCMG,FonteFP,etal.Avaliac¸ãodonúmerode célulascaliciformesnascriptasdamucosacolônicacome semtrânsitointestinal.RevColBrasCir.2012;39:
139–45.
28.MartinezCAR,FabrisFM,SilvaCMG,etal.Oxidativestress andchangesinthecontentandpatternoftissueexpression of-cateninproteinindiversioncolitis.JColoproctol(RioJ). 2012;32:343–58.
29.KadriCJ,PereiraJA,SilvaCM,etal.E-cadherinexpressionin colonicmucosawithandwithoutfecalstream.JInvestSurg. 2013;26:72–9.
30.LameiroTMM,SilvaCMG,MarquesLHS,etal.Efeitosdo butiratonosníveisdeperoxidac¸ãolipídicaemcélulasda mucosacólicasemtrânsitofecal:estudoexperimentalem ratos.RevBrasColo-proctol.2011;31:
155–64.
31.CunhaFL,SilvaCMG,AlmeidaMG,etal.Reductionin oxidativestresslevelsinthecolonicmucosawithoutfecal streamaftertheapplicationofenemascontainingaqueous Ilexparaguariensisextract.ActaCirBras.2011;26:
289–96.
33.RileySA,GuptaI,ManiV.Acomparisonofsucralfateand prednisoloneenemasinthetreatmentofactivedistal ulcerativecolitis.ScandJGastroenterol.1989;24:1014–8. 34.LaudannoOM,BediniOA,CesolariJA,SanMiguelP.Evidence
ofanti-oxidantroleofsucralfateingastricmucosal protection.ItalJGastroenterol.1990;22:19–21.
35.Al-SwayehOA,al-HumayydMS,MustafaAA,al-TuwaijriAS, al-RashedRS,AliAT.Sucralfateattenuatesgastricmucosal lesionsandincreasedvascularpermeabilityinducedby ischaemiaandreperfusioninrats.JGastroenterolHepatol. 1997;12:481–9.
36.ZahaviI,AvidorI,MarcusH,etal.Effectofsucralfateon experimentalcolitisintherat.DisColonRectum. 1989;32:95–8.
37.BjörckS,JennischeE,DahlströmA,AhlmanH.Influenceof topicalrectalapplicationofdrugsondextransulfate-induced colitisinrats.DigDisSci.1997;42:824–32.
38.LongattiTS,AcedoSC,deOliveiraCC,etal.Inflammatory alterationsinexcludedcoloninrats:acomparisonwith chemicallyinducedcolitis.ScandJGastroenterol. 2010;45:315–24.