w ww.e l s e v i e r . c o m / l o c a t e / b j p
Short
communication
Chrysosplenetin,
in
the
absence
and
presence
of
artemsininin,
alters
breast
cancer
resistance
protein-mediated
transport
activity
in
Caco-2
cell
monolayers
using
aristolochic
acid
I
as
a
specific
probe
substrate
Yuanyuan
Zhang
a,1,
Chenxu
Zhang
a,1,
Jie
Chen
a,
Liping
Ma
a,
Bei
Yang
a,
Jianhuan
Wang
a,
Xiuli
Wu
a,b,c,∗,
Jing
Chen
a,b,c,∗aCollegeofPharmacy,NingxiaMedicalUniversity,Yinchuan,China
bNingxiaEngineeringandTechnologyResearchCenterforModernizationofHuiMedicine,Yinchuan,China
cKeyLaboratoryofHuiEthnicMedicineModernization,MinistryofEducation,NingxiaMedicalUniversity,Yinchuan,China
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received12July2017 Accepted16October2017 Availableonline16November2017
Keywords:
Chrysosplenetin Artemisinin AristolochicacidI
Breastcancerresistanceprotein Caco-2cellsmonolayers
a
b
s
t
r
a
c
t
Thepresentstudydescribestheimpactofchrysosplenetin,intheabsenceandpresenceofartemisinin, oninvitrobreastcancerresistanceprotein-mediatedtransportactivityinCaco-2cellmonolayersusing aristolochicacidIasaspecificprobesubstrate.Weobservedthatnovobiocin,aknownbreastcancer resistanceproteinactiveinhibitor,increasedPapp(AP-BL)ofaristolochicacidI3.13fold(p<0.05)buthad
noeffectonPapp(BL-AP).Effluxratio(PBA/PAB)declined4.44fold(p<0.05).Novobiocin,consequently,
showedadirectfacilitationontheuptakeofAAIinsteadofitsexcretion.Oppositely,bothartemisininand chrysosplenetinaloneatdoseof10MsignificantlydecreasedPapp(BL-AP)insteadofPapp(AP-BL).
Chrysos-plenetinaloneattenuatedtheeffluxratio,whichwassuggestiveofbeingasapotentialbreastcancer resistanceproteinsuppressant.Oddly,Papp(BL-AP)aswellaseffluxratiowererespectivelyenhanced2.52
and2.58fold(p<0.05),whenco-usedwithartemisininandchrysosplenetininratioof1:2.Thepotential reasonremainsunclear;itmightberelativetobindingsitescompetitionbetweenartemisininand chrysosplenetinorthehomodimer/oligomerformationofbreastcancerresistanceproteinbridgedby disulfidebonds,leadingtoanalteredinvitrobreastcancerresistanceprotein-mediatedeffluxtransport function.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
ArtemisininresistanceinPlasmodiumfalciparum,characterized byslowparasitesclearanceinpatientsreceivingartemisininoran artemisinin-basedcombinationtherapy(ACT),wasfirstdetected along the Thai–Cambodian border and has been spread across mainlandSoutheastAsia(Noedletal.,2008;Dondorpetal.,2009; Amaratungaetal.,2012;Ashleyetal.,2014;Imwongetal.,2017). The resistantmechanism to artemisinin is still ambiguous and manymultidrugresistanceproteinsprobablyinvolvedin,suchas P-glycoprotein(P-gp),breastcancerresistanceprotein(BCRP),bile
∗ Correspondingauthors.
E-mails:xiuli2005@163.com(X.Wu),chenjing@nxmu.edu.cn(J.Chen).
1Theseauthorscontributedequallytothiswork.
saltexportpump(BSEP),andmultidrugresistance-associated pro-teins(MRP)1–4(Alcantaraetal.,2013;Rijpmaetal.,2014).
BCRPbelongs totheABCtransporterfamily(Allikmetsetal., 1998;DoyleandRoss,2003)withaC-terminaltransmembrance domain and an N-terminal ATP-binding domain(Litman et al., 2000),consistingof655aminoacids(72-kDa).Therefore,BCRPisa halftransporterthattransformstoafunctionaleffluxpumpwhen homodimerizedbyadisulfidebridgeatCys603oftwoproteins (Lecerf-Schmidtetal.,2013;Noguchietal.,2014;MaoandUnadkat, 2015)andconfersanatypicalMDRphenotype.
Inourpreviouswork,wefoundchrysosplenetin,aknown poly-methoxylatedflavonoidsinArtemisiaannuaL.,incombinationwith artemisinin(2:1)decreasedBcrp/ABCG2mRNAexpressionlevels inmicesmallintestine(datanotshown).Therefore,wehereaimed tofurtherinvestigatetheeffectofchrysosplenetinintheabsence orpresence of artemisininoninvitroBCRP-mediated transport
https://doi.org/10.1016/j.bjp.2017.10.006
activitybyusingAAIasaspecificprobesubstrateinCaco-2cell monolayers.
Materialsandmethods
Artemisinin was purchased from Chongqing Huali Konggu Co.,Ltd.(Chongqing,China)withpurity≥99.0%.Chrysosplenetin (purity ≥98.0%) waspurified in our lab from an acetone layer ofwastematerialsinartemisininindustrialproductionbyusing multiple column chromatography methods as described in the literature(Weietal.,2015).Thewastematerialswerekindly sup-pliedbyChongqingHualiKongguCo.,Ltd.Thevoucherspecimen (20100102)hasbeendepositedwithCollegeofPharmacy,Ningxia Medical University,for furtherreferences.Novobiocin was pur-chasedfromHefeiBomeiBiotechnologyCo.,Ltd.(CAS:1476-53-5, purity≥90%,China).BotharistolochicacidI(AAI,110746-201510, purity≥98%)andindomethacin(I.S.,100258-200904,purity≥99%) werepurchasedfromNationalInstitutesforFoodandDrugControl (China).
Methanoland acetonitrile(HPLC-grade)waspurchasedfrom Tedia(Ohio,USA).MTT(thiazolyl blue)andLuciferyellowwere purchasedfromSigma–AldrichCo. Ltd.(USA).HBSS(Hanks Bal-ancedSaltSolution)wasprovidedbyKangweiShijibiotechnology Co.Ltd.(H1020,China).
AnAgilentHPLC1200systemwasusedforthedetermination of AAI. Samples wereseparated with a Zorbax SB-C18 column (4.6×250mm,5m,AgilentTechnologies,USA).AAI concentra-tionwasanalyzedbyRP-HPLC-UVassayaccordingtothereported methodwithsomemodification(Kimura etal.,2014;Maetal., 2015).Mobilephaseconsistedof45%ofacetonitrileand55%of1% aceticacidinwater.Columntemperaturewassetat30◦Candflow ratewassetat1.0ml/min.AAIwasdetectedatthewavelengthof 250nm.Theinjectionvolumewas20l.
Standard stock solutions of AAI and indometacin (I.S.) were individuallypreparedinmethanolatconcentrationof40Mand 2.80M,andstoredat4◦Cbeforeuse.Standardworkingsolutions werepreparedbydilutingthestocksolutioninmethanoltoobtain aserialofdesiredconcentrations.Chrysosplenetinandartemisinin wererespectivelydissolvedinDMSO(dimethylsulfoxide)inthe strengthof100mM.Allthesolutionswerestoredat−20◦Cand broughttoroomtemperaturebeforeuse.
The chromatographic method was validated for specificity, linearity,sensitivity, precisionand accuracy.All validation runs wereperformedin five replicatesonthree consecutivedaysto assessinter-dayandintra-dayvariation.Calibrationcurvewas con-structedfortherange0,1,2,5,10,20,50,100M.BlankCaco-2 monolayerbuffersamples(n=5)wereinjectedforspecificitytest. Precisionandaccuracywasassessedatthreeconcentrations,i.e. low (LQC,5M), medium(MQC,20M) and highquality con-trols(HQC,100M).Itwasfurthersubdividedintointra-dayand inter-dayprecision.Thelowestlimitofquantification(LLOQ)was determinedbyserialdilutionofworkingstandards.
Stability experiments were performed under different con-ditionsbysimulatingconditions occurringduringstudysample analysis.Experimentsweremanipulatedtodeterminethestability at37◦Cfor3h,ambienttemperaturefor4h,and−20◦Cfor7days. Caco-2cells(Fig.1)wereseededinthetranswell polycarbo-nateinsertsatadensityof106cellsperwellandweregrownina culturemediumconsistingofDulbecco’smodifiedEagle’smedium (DMEM/F-12)supplemented with10%fetal bovineserum(FBS), 1%nonessentialaminoacids,1%l-glutamine,100U/ml penicillin-Gand100g/mlstreptomycin.Theculturemediumwasreplaced everyalternatedayandthecellsweremaintainedat37◦C,95% rel-ativehumidityand5%CO2.Permeabilitystudieswereconducted withthemonolayersculturedfor19–21days.
Fig.1.Caco-2cellsgrowthsituation.
Toensure themonolayerintegrity throughout thecourseof transportexperiment, transepithelialelectricalresistance-values (TEERvalues)weremeasuredwithaMillicell-ERSVolt-ohmmeter. Apparentpermeabilitycoefficient(Papp)ofLuciferyellow,which alwaysusedasamarkerofparacellulartransport,wasalso deter-mined.TheintegrityofmonolayerswasconfirmedwhentheTEER values ofCaco-2cells exceeded400/cm2,and theP
app values of Lucifer yellow were less than 0.5×10−6cm/s ( Aspenström-Fagerlundetal.,2012).
ToensuretheproperconcentrationsofAAIusedintheuptake andtranscellulartransportstudy,thecytotoxicityeffectwas eval-uatedbyMTTassay.Caco-2cellswereseededinto96-wellplate atadensityof1.0×104cells/well.AAIwereaddedatdesignated concentrationsfollowedby4hincubationat37◦C.After remov-ingthemedium,theserum-freemediumcontaining15lofMTT (5.0mg/ml)wasaddedandincubatedforanother4h.Intheend, 200lofDMSOreplacedtheMTTmediumtodissolveformazan, andthentheabsorbanceatawavelengthof490nmwasmeasured bySpectraMaxM2microplatereader.
Before the experiments, Caco-2 cells were divided into five groups including negative control (HBSS), artemisinin alone (10M),novobiocin(positivecontrol, 100M),chrysosplenetin (10M),andartemisinin–chrysosplenetin(1:2).Cellmonolayers werewashedtwicewithwarmHBSSandequilibratedwithHBSS for30minat37◦C.Thetransportstudieswereinitiatedby load-ingtheindividualHBSSsolutionofAAI(90M) ontothedonor compartment.Theothersidewastermedthereceiver compart-ment,and thefinal volumeineachof thechamberswas2.5ml ontheapicalside(AP)and2.5mlonthebasolateralside(BL).A 200laliquotofsampleswasseparatelytakenoutfromthedonor and receiver chambers each 30min till 2hand fresh transport mediumwasimmediatelycomplemented.Transportexperiments wereconductedinanincubatormaintainedat37◦Candshaken withaspeedof50rpm.A200laliquotofcelllysateswasused forextractionat0,30,60,90,and120min.Total100lofthe har-vestedsamplewasaddedinto1mlethylacetatecontaining2g/ml ofindomethacin(I.S.),vortex-mixedfor3minandthencentrifuged at13,800×gfor10min.Andthen850lofsupernatantwas evapo-ratedtodrynessundervacuumandtheresiduewasresuspendedin 200lofmobilephase.Afterbeingvortex-mixedandcentrifuged at13,800×gfor10min,thesupernatantwasusedtodetermine AAIconcentrationbyanestablishedRP-HPLC-UVmethod.
Table1
ApparentpermeabilitycoefficientofLucieryellow.
No. Fluorescenceintensity Concentration (g/ml)
Papp (cm/s)
1 103.26 0.012 4.04×10−7
2 102.68 0.009 3.03×10−7
3 101.97 0.005 1.68×10−7
-5 0 5 10 15
DMSO 10 30 60 90 120 150
Inh
ibit
ion r
a
ti
o
(%
)
AAI concentration (µmol/l)
Fig.2. ThecytotoxicityeffectofAAIontheviabilityofCaco-2cells.
Significantdifferenceswereatp<0.05orp<0.01levelbetweenthe groups.Turkey’stestwasusedtoidentifyanydifferencebetween meansusingasignificancelevelofp<0.05.
Resultsanddiscussion
TheTEERvaluesofCaco-2celllinesweredeterminedtobeover 420×cm2(454,430,and476×cm2).Apparentpermeability coefficientsofLucieryellowdisplayedinTable1revealeda nor-maltransportpathwayinestablishedCaco-2cellmonolayermode, withoutaleakageofLucieryellow.Meanwhile,MTTcytotoxicity test(showedinFig.2)indicatedthatnoremarkablepoisonouswas observedunder90MofAAIwithin3h.
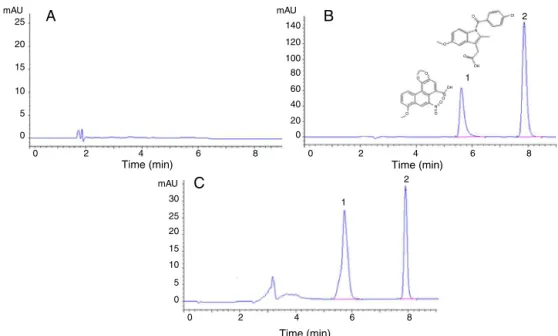
Fig.3showstherepresentativechromatogramsofblankHBSS buffer,standardsubstancesspikedinHBSSbuffer,andsample har-vestedafter1h.InjectionofblanktransportbufferontotheHPLC columnshowednointerference.
ThecalibrationcurveofAAIpresentedawelllinearityinthe range0–100Mwhenspikedin blankHBSSbuffer.The regres-sionequationforcalibrationcurveswasY=1.01X(r=0.99996).The
intra-andinter-dayprecisions(%CV)werelessthan3.10%and accu-racies(%RE)werewithin-0.32%and3.20%(Table2).TheLLOQofAAI wasfoundtobe1M.Thisindicatedthatthemethodwasfeasible fortheanalysisofAAI.
StabilityresultsweredescribedinTable3.Itshowedthatthe analyteswerestableafterbeingplacedat37◦Cfor3h, ambient temperaturefor4h,and−20◦Cstoredfor7days.
AsshowninTable4,theeffluxratioofAAIinCaco-2cell mono-layersin negativecontrolgroupwas6.80, whichindicated that effluxtransportersmightbeinvolvedinthetransportofAAI.Itis inaccordancewiththeliterature(Maetal.,2015).Papp(AP-BL)value ofAAIsignificantlyincreased3.13foldswhenco-usedwith posi-tivecontrolnovobiocin(p<0.05).Nosignificancewasobservedin Papp(BL-AP)value(p>0.05)buttheeffluxratio(PBA/PAB)was drasti-callydecreased4.44folds(p<0.05).Novobiocin,therefore,mainly showedadirect promotionontheuptake ofAAIinsteadofthe inhibitionofBCRP-mediatedAAIefflux.
BothchrysosplenetinandartemisininalonedeclinedPapp(BL-AP) whilehavenoimpactonPapp(AP-BL) relativetonegativecontrol (p<0.05).Moreover,chrysosplenetinsignificantlyattenuatedthe effluxratio.Itimpliedthatchrysosplenetininhibitedtheinvitro BCRP-mediatedeffluxofAAIinCaco-2cellmonolayerswhen inde-pendentlyused.However,whencombinedwithartemisininand chrysosplenetininratioof1:2,Papp(BL-AP)andeffluxratiowere significantlyincreased2.52-and2.58-fold(p<0.05)alongwithan unchangedPapp(AP-BL).
Inconclusion, BCRP-mediatedeffluxof AAIwasinhibitedby chrysosplenetinalonewhileremarkablypromotedwhenco-used withartemisinin.Thepotentialreasonhasnotbeenfully under-stood.BCRPpossessesmultipledrugbindingsitesinalargepocket formedbyTM˛-helices.SomeinhibitorsasBCRPsubstratescanact ascompetitiveinhibitors.Inthisregard,itispossiblethat chrysos-plenetinandartemisinininteractwithBCRPonbindingsitesand induceconformationalchangesin thelargebindingpocket, and thusallostericallyaffecttheeffluxtransportofAAIasaspecificBCRP substrate.Secondly,BCRPisassumedtoactasafunctional homod-imerbridgedbydisulfidebonds(Kageet al.,2002)or oligomer (Kage et al.,2005).This deservesa further worktoinvestigate whetherchrysosplenetin in thepresenceof artemisinin altered
25 mAU
A
C
B
mAU
mAU
25
140
30
120
100
80
60
40
20
0 20
20 15
15 10
10 5
5 0
0
0 0
0
1
1
2
2
2 2
2
4 4
4
Time (min) Time (min)
Time (min)
6 6
6
8 8
8
Table2
MethodvalidationfortheanalysisofAAI(Mean±SD,n=5).
Theoreticalconcentrationa(mol/l) Accuracyandprecision Averageextractionrecoveries(%)
Intra-day Inter-day
Measured concentration (g/l)
Precision (%CV)
Accuracy (%RE)
Measured concentration (g/l)
Precision (%CV)
Accuracy (%RE)
5 5.04±0.03 0.60 0.80 5.16±0.16 3.10 3.20 70.80±0.32 20 20.13±0.21 1.04 0.65 20.33±0.43 2.12 1.65 72.40±0.07 100 99.68±0.90 0.90 −0.32 100.15±1.90 1.90 1.50 78.30±0.13
aSelectedconcentrationsrepresentthelow,mediumandhigh(LQC,MQC,andHQC,respectively)concentrations.
Intra-dayandinterdayaccuracyandprecisionweredeterminedwithn=5foreachconcentration.
%RE(%relativeerror)=[(Measuredconcentration−theoreticalconcentration)/theoreticalconcentration]×100. %CV(%coefficientofvariation)=(SD/mean)×100.
Table3
StabilitystudiesforAAIunderdifferentstorageconditions(n=5).
Condition Qualitycontrola
(M)
Measuredconcentration (ng/ml)
Precision (%CV)
Accuracy (%RE)
37◦Cfor3h 5 5.08±0.24 4.72 1.60
20 20.40±1.35 6.62 2.00
100 110.35±3.56 3.23 10.35
Roomtemperaturefor4h 5 5.19±0.32 6.17 3.80
20 20.35±2.02 9.93 1.75
100 111.45±4.15 3.72 11.45
−20◦Cfor7days 5 4.78±0.22 4.60 −4.40
20 18.43±0.35 1.90 −7.85
100 89.18±3.23 3.62 −10.82
aSelectedconcentrationsrepresentthelow,mediumandhigh(LQC,MQC,andHQC,respectively)concentrations.
Datawereexpressedasmean±SD(n=5).
%RE(%relativeerror)=[(Measuredconcentration−theoreticalconcentration)/theoreticalconcentration]×100. %CV(%coefficientofvariation)=(SD/mean)×100.
Table4
ChrysosplenetinalterspermeabilityofAAIacrossCaco-2cellmonolayersintheabsenceorpresenceofartemisinin(mean±SD,n=3).
Drugs Dosage
(M)
Papp (×10−5cm/s)
PBA/PAB (effluxratio)
AP-BL BL-AP
Negativecontrol – 3.35±0.11 22.78±0.00 6.80±0.21
Novobiocin(positivecontrol) 100 10.5±1.46a↑ 16.6±0.28 1.53±0.19a↓
Artemisininalone 10 2.49±0.12 15.2±1.41a↓ 6.09±0.87
Chrysosplenetinalone 10 3.08±0.06 15.5±0.28a↓ 5.04±0.01a↓
Artemisinin:Chrysosplenetin(1:2) 10:20 3.28±0.03 57.5±8.62a↑ 17.52±2.58a↑
ap<0.05vsnegativecontrol.
BCRPhomodimer/oligomerlevelswhichmightleadtotheadverse resultinthisstudy.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat nopatientdataappearinthisarticle.
Author’scontributions
YZandCZcontributedinrunningthelaboratorywork.JCand LManalyzedthedata.BYandJWrevisedthemanuscriptcritically.
XWandJCdesignedthestudy,supervisedthelaboratoryworkand contributedtomodifythemanuscript.Alltheauthorshaveread thefinalmanuscriptandapproveditssubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
ThisworkwasfinanciallysupportedbytheNationalNatural Sci-enceFoundationofChina(81560580)andNingxiaKeyResearch Projects(2015BN24).
References
Alcantara,L.M.,Kim,J.,Moraes,C.B.,Franco,C.H.,Franzoi,K.D.,Lee,S.,Freitas-Junior, L.H.,Ayong,L.S.,2013.ChemosensitizationpotentialofP-glycoproteininhibitors inmalariaparasites.Exp.Parasitol.134,235–243.
Amaratunga,C.,Sreng,S.,Suon,S.,Phelps,E.S.,Stepniewska,K.,Lim,P.,Zhou,C., Mao,S.,Anderson,J.M.,Lindegardh,N.,Jiang,H.,Song,J.,Su,X.Z.,White,N.J., Dondorp,A.M.,Anderson,T.J.,Fay,M.P.,Mu,J.,Duong,S.,Fairhurst,R.M.,2012.
Artemisinin-resistantPlasmodiumfalciparuminPursatprovince,western Cam-bodia:aparasiteclearanceratestudy.LancetInfect.Dis.12,851–858.
Ashley,E.A.,Dhorda,M.,Fairhurst,R.M.,Amaratunga,C.,Lim,P.,Suon,S.,Sreng,S., Anderson,J.M.,Mao,S.,Sam,B.,Sopha,C.,Chuor,C.M.,Nguon,C.,Sovannaroth, S.,Pukrittayakamee,S.,Jittamala,P.,Chotivanich,K.,Chutasmit,K., Suchatsoon-thorn,C.,Runcharoen,R.,Hien,T.T.,Thuy-Nhien,N.T.,Thanh,N.V.,Phu,N.H., Htut,Y.,Han,K-T.T.,Aye,K.H.,Mokuolu,O.A.,Olaosebikan,R.R.,Folaranmi,O.O., Mayxay,M.,Khanthavong,M.,Hongvanthong,B.,Newton,P.N.,Onyamboko, M.A.,Fanello,C.I.,Tshefu,A.K.,Mishra,N.,Valecha,N.,Phyo,A.P.,Nosten,F., Yi,P.,Tripura,R.,Borrmann,S.,Bashraheil,M.,Peshu,J.,Faiz,M.A.,Ghose,A., Hossain,A.,Samad,R.,Rahman,R.,Hasan,M.M.,Islam,A.,Miotto,O.,Amato, R.,MacInnis,B.,Stalker,J.,Kwiatkowski,D.P.,Bozdech,Z.,Jeeyapant,A.,Cheah, P.Y.,Sakulthaew,T.,Chalk,J.,Intharabut,B.,Silamut,K.,Lee,S.J.,Vihokhern,B., Kunasol,C.,Imwong,M.,Tarning,J.,Taylor,W.J.,Yeung,S.,Woodrow,C.J.,Flegg, J.A.,Das,D.,Smith,J.,Venkatesan,M.,Plowe,C.V.,Stepniewska,K.,Guerin,P.J., Dondorp,A.M.,Day,N.P.,White,N.J.,2014.Spreadofartemisininresistancein Plasmodiumfalciparummalaria.N.Engl.J.Med.371,411–423.
Aspenström-Fagerlund,B.,Tallkvist,J.,Ilbäck,N.G.,Glynn,A.W.,2012.Oleicacid decreasesBCRPmediatedeffluxofmitoxantroneinCaco-2cellmonolayers.Food Chem.Toxicol.50,3635–3645.
Dondorp,A.M.,Nosten,F.,Yi,P.,Das,D.,Phyo,A.P.,Tarning,J.,Lwin,K.M.,Ariey,F., Hanpithakpong,W.,Lee,S.J.,Ringwald,P.,Silamut,K.,Imwong,M.,Chotivanich, K.,Lim,P.,Herdman,T.,An,S.S.,Yeung,S.,Singhasivanon,P.,Day,N.P., Linde-gardh,N.,Socheat,D.,White,N.J.,2009.ArtemisininresistanceinPlasmodium falciparummalaria.N.Engl.J.Med.361,455–467.
Doyle,L.,Ross,D.D.,2003.Multidrugresistancemediatedbythebreastcancer resis-tanceproteinBCRP(ABCG2).Oncogene22,7340–7358.
Imwong,M.,Suwannasin,K.,Kunasol,C.,Sutawong,K.,Mayxay,M.,Rekol,H., Smithuis,F.M.,Hlaing,T.M.,Tun,K.M.,vanderPluijm,R.W.,Tripura,R.,Miotto, O.,Menard,D.,Dhorda,M.,Day,N.P.J.,White,N.J.,Dondorp,A.M.,2017.The spreadofartemisinin-resistantPlasmodiumfalciparumintheGreaterMekong subregion:amolecularepidemiologyobservationalstudy.LancetInfect.Dis.17, 491–497.
Kage,K.,Tsukahara,S.,Sugiyama,T.,Asada,S.,Ishikawa,E.,Tsuruo,T.,Sugimoto,Y., 2002.Dominant-negativeinhibitionofbreastcancerresistanceproteinasdrug effluxpumpthroughtheinhibitionofS-Sdependenthomodimerization.Int.J. Cancer.97,626–630.
Kage,K.,Fujita,T.,Sugimoto,Y.,2005.RoleofCys-603indimer/oligomer for-mationofthebreastcancerresistanceproteinBCRP/ABCG2.CancerSci.96, 866–877.
Kimura,O.,Haraguchi,K.,Ohta,C.,Koga,N.,Kato,Y.,Endo,T.,2014.Uptakeof aris-tolochicacidIintoCaco-2cellsbymonocarboxylicacidtransporters.Biol.Pharm. Bull.37,1475–1479.
Lecerf-Schmidt,F.,Peres,B.,Valdameri,G.,Gauthier,C.,Winter,E.,Payen,L.,DiPietro, A.,Boumendjel,A.,2013.ABCG2:recentdiscoveryofpotentandhighlyselective inhibitors.FutureMed.Chem.5,1037–1045.
Litman,T.,Brangi,M.,Hudson,E.,Fetsch,P.,Abati,A.,Ross,D.D.,Miyake,K.,Resau, J.H.,Bates,S.E.,2000.Themultidrug-resistantphenotypeassociatedwith over-expressionofthenewABChalf-transporter,MXR(ABCG2).J.Cell.Sci.113, 2011–2021.
Ma,L.P.,Qin,Y.H.,Shen,Z.W.,Bi,H.C.,Hu,H.Y.,Huang,M.,Zhou,H.,Yu,L.S.,Jiang,H.D., Zeng,S.,2015.AristolochicacidIisasubstrateofBCRPbutnotP-glycoprotein orMRP2.J.Ethnopharmacol.172,430–435.
Mao, Q., Unadkat, J.D., 2015. Role of the breast cancer resistance protein (BCRP/ABCG2)indrugtransport–anupdate.AAPSJ.17,65–82.
Noedl,H.,Se,Y.,Schaecher,K.,Smith,B.L.,Socheat,D.,Fukuda,M.M.,2008. Evi-denceofartemisinin-resistantmalariainwesternCambodia.N.Engl.J.Med. 359,2619–2620.
Noguchi,K.,Katayama,K.,Sugimoto,Y.,2014.HumanABCtransporterABCG2/BCRP expressioninchemoresistance:basicandclinicalperspectivesformolecular cancertherapeutics.PharmgenomicsPers.Med.7,53–64.
Rijpma,S.R.,vandenHeuvel,J.J.,vanderVelden,M.,Sauerwein,R.W.,Russel,F.G., Koenderink,J.B.,2014.Atovaquoneandquinineanti-malarialsinhibitATP bind-ingcassettetransporteractivity.Malar.J.13,359–366.