UNILATERAL SUBTHALAM IC NUCLEUS LESIONING
A safe and effect ive t reat ment for Parkinson` s disease
Osvaldo Vilela Filho, Délson José da Silva
ABSTRACT - The present st udy, t he largest in t he lit erat ure, w as performed t o assess t he effect iveness and safet y of unilat eral subt halamic nucleus (STN) lesioning for Parkinson’s disease (PD). From August 1999 t o September 2000, 21 consecutive patients evaluated pre- and postoperatively by a single examiner w ere operated. Levodopa int ake and dyskinesia, Hoehn & Yahr, Schw ab & England and UPDRS mot or scores w ere recorded. St ereot act ic CT and M RI and t he effect s of macrost imulat ion w ere used t o det ermine STN coordinat es. A single radiofrequency lesion w as made (60-75ºC/60” ). Concomit ant ipsilat eral Vim/VOp lesions w ere made in 8 pat ient s. Using a new t echnique, w e w ere able t o det ermine t he t errit ory of STN involved by t he surgical lesion. The Wilcoxon and M ann-Whit ney st at ist ical t est s w ere applied t o evaluat e t he surgical result s. All recorded paramet ers show ed st able improvement aft er a mean follow up of 13.5 mont hs. Recurrence occurred in t w o pat ient s. Cont ralat eral t remor arrest and decrease of rigidit y and bradykinesia should be regarded as STN hallmarks t o st imulat ion. Hyperint ense lesions in t he early-phase M RI seem t o be a poor prognost ic fact or. Lat eral t errit ory lesioning correlat es w it h bet t er result s. There w as no significant difference bet w een t he cohort s w it h and w it hout a Vim/VOp lesion. Dyskinesias happened in t w o pat ient s (prompt ly abolished by a Vim/VOp lesion). Ot her complicat ions w ere t ransient and/or rare. In conclusion, STN lesioning is a safe and very effect ive procedure t o t reat PD and probably an underut ilized operat ion for t hose w ho can not afford t he cost s of DBS.
KEY WORDS: subt halam ic nucleus lesioning, deep brain st im ulat ion, st ereot act ic surgery, subt halam ic nucleot omy, subt halamot omy, Parkinson’s disease.
Lesão unilateral do núcleo subtalâmico: um tratamento seguro e eficaz para a doença de Parkinson
RESUM O - O present e est udo, o maior da lit erat ura, foi realizado para avaliar a eficácia e segurança da lesão unilat eral do NST (núcleo subt alâmico) para o t rat ament o da DP (doença de Parkinson). Ent re agost o de 1999 e set embro de 2000, 21 pacient es consecut ivos avaliados pré e pós-operat oriament e por um único examinador foram operados. Os seguint es parâmet ros foram avaliados: dose diária de levodopa, discinesia induzida pela levodopa, est adiam ent o da doença, at ividades de vida diária e escores m ot ores da UPDRS. RNM e TC est ereot áxicas e os ef eit os da est im ulação com m acroelet rodo f oram ut ilizados na det erm inação das coordenadas do NST. Uma única lesão por radiofrequência foi realizada (60 – 75ºC / 60” ). Lesão concomit ant e de Vim/VOp ipsilat eral foi realizada em 8 pacient es. Ut ilizando-se uma nova t écnica, foi possível est abelecer o t errit ório do NST lesado cirurgicament e. Os t est es est at íst icos de Wilcoxon e M ann-Whit ney foram aplicados para avaliar os result ados cirúrgicos. Todos os parâmet ros avaliados apresent aram melhora sust ent ada após seguiment o médio de 13.5 meses. Recidiva ocorreu em 2 casos. Abolição do t remor e redução da rigidez e bradicinesia cont ralat erais devem ser considerados como “ marcadores” do NST à est imulação. A presença de lesão hiperint ensa na RNM pós-operat ória precoce parece ser indicadora de mau prognóst ico. Os melhores result ados foram obt idos com a lesão do t errit ório lat eral do NST. Não houve diferença est at ist icament e significant e ent re os subgrupos com e sem lesão concomit ant e de Vim /VOp. Discinesia ocorreu em 2 casos, pront ament e revert ida pela lesão concomit ant e de Vim/VOp. Out ras complicações foram t ransit órias e/ou raras. Concluindo-se, a lesão unilat eral do NST é procediment o seguro e eficaz para o t rat ament o da DP, tratando-se, de uma cirurgia subutilizada para aqueles que não podem arcar com o elevado custo da estimulação cerebral profunda.
PALAVRAS-CHAVE: núcleo subt alâm ico, lesão, est im ulação cerebral prof unda, cirurgia est ereot áxica, nucleot omia subt alâmica, subt alamot omia, doença de Parkinson.
St ereot act ic and Funct ional Neurosurgery Service and Parkinson’s Disease and M ovement Disorders Unit of Hospit al das Clínicas, M edical School, Universidade Federal de Goiás, and t he St ereot act ic and Funct ional Neurosurgery Service of t he Inst it ut o do Cérebro de Goiânia, Hospit al Lúcio Rebelo, Goiânia GO, Brazil.
Received 6 June 2002, received in final form 30 August 2002. Accept ed 2 Sept ember 2002.
Despit e having been performed for more t han 70 years1, it w as only in t he last decade of t he last
cent ury, aft er t he development of non-human pri-mate animal models of M PTP-induced parkinsonism2,
t hat t he surgical t reat ment of Parkinson’s disease (PD) abandoned t he empiric field t o become really scient ific. Using t hese models it w as possible t o de-monst rat e hyperact ivit y in t he globus pallidus in-t ernus (GPi) and subin-t halamic nucleus (STN) and in-t he presence of tremor-cells in GPi and STN1,3-7, validating
previously proposed circuit s of t he basal ganglia8-10.
Such know ledge made possible t he comprehension of the genesis of the cardinal manifestations of PD1,3-7
and how t o bet t er t reat t hem surgically1. Besides, a
new hypot hesis has been advanced: t he excit ot oxic effect of STN glut amat e on dopaminergic cells of subst ant ia nigra pars compact a (SNC) w ould act as a perpet uat ing mechanism of dopaminergic cell de-at h and PD progression3,4,7,11.
Taking t hese f indings all t oget her, one could suppose that STN w ould be the most suitable target to treat PD3, 4. STN deep brain stimulation (STN-DBS)
w as first performed by Benabid’s group in 199312,13.
Since then, many groups, including his ow n, have re-plicated the excellent results initially reported7,14-25. STN
lesioning, on the other hand, w as first reported by Obeso et al. in 199726. Probably due to the fear of
producing hemiballismus, this procedure has been re-ported in only 21 patients all over the world1,3,4,7,14,17,26-30,
despite the significant improvement obtained. The present aut hors, in an at t empt t o det ermine t he safet y and efficacy of unilat eral STN lesioning, prospect ively performed t his procedure in 23 con-secut ive pat ient s w it h idiopat hic PD, regardless t he predominant manifest at ions of t he disease. To t he best of our know ledge, t he present st udy represent s t he largest series on STN lesioning for PD t reat ment report ed in t he lit erat ure.
M ETHOD
From August 1999 t o Sept ember 2000, aft er informed consent , 23 consecut ive pat ient s w it h PD underw ent uni-lat eral STN lesioning in our t w o Services (Hospit al das Clí-nicas of Universidade Federal de Goiás and Inst it ut o do Cérebro de Goiânia). Tw o pat ient s w ere excluded from analysis due t o event s not relat ed t o t he operat ion it self, prevent ing an adequat e evaluat ion of t he surgical result . Of t he remaining 21 pat ient s, all present ed bilat eral disease. There w ere 16 male and 5 female, mean age of 56 years (38 t o 77 years; ≤ 60 years = 11 pat ient s and > 60 years = 10 pat ient s) and mean durat ion of t he disease of 8.4 years (2.5 t o 19 years; ≤ 5 years = six pat ient s, 6-9 years = five pat ient s and ≥ 10 years = nine pat ient s and u n kn o w n = o n e p at ien t ). Th ree p at ien t s h ad b een
previously operat ed cont ralat erally t o t he proposed sur-gery, w it h good result s: Vim/VOp (int ermediat e vent ral nucleus / post erior vent ral oral nucleus) t halamot omy, one pat ient and campot omy, t w o pat ient s.
All pat ient s w ere evaluat ed pre and post operat ively, w hile “ on” , by a single examiner (DJS). Levodopa int ake [post operat ively it w as classified as: unchanged, slight re-duct ion (33%) or significant rere-duct ion (≥ 50%)], levodopa-induced dyskinesia, Hoehn & Yahr M odified Scale (H&Y), Schw ab & England Scale (S&E) and UPDRS (Unified Par-kinson’s Disease Rat ing Scale) mot or scores w ere recorded bilat erally, but only t hose cont ralat eral t o t he surgery w ere consid ered f or analysis (Tab le 1). Post op erat ively, all pat ient s w ere asked t o self-report t heir improvement .
Indications and contraindications for surgical treatment w ere already report ed elsew here1,3,31.
All pat ient s w ere operat ed by one of t he aut hors (OVF). Under local anest hesia, t he st ereot act ic frame (model M T-03B, M icromar St ereot act ic Syst em, São Paulo, Brazil) is placed parallel t o t he infraorbit al – ext ernal audit ory canal line [t his line is usually parallel t o t he AC-PC (ant eri-or commissure-post erieri-or commissure) line], t he box con-t aining con-t he M RI (magnecon-t ic resonance imaging) fiducials is adapt ed and an M RI (Gyroscan Nt 10, 1 t esla, Philips) is performed (15 of our patients) obeying the protocol show n in Table 2, t aking care t o place t he probable project ion of t he AC-PC line (usually 2 cm above t he nasion) as close as possible t o t he cent er of t he magnet ic field. The axial slices are performed parallel t o t he frame and t he coronal slices, perpendicular t o it , t he cent ral one passing t hrough t he midcommissural point (M CP). The coordinat es of AC, PC and M CP are obt ained from t he axial slices using t he resi-dent soft w are of t he M RI.
Fort unat ely, STN can usually be seen on T2-w eight ed coronal images. The landmarks for it s ident ificat ion are show n in Figure 1A. The t arget is chosen in it s cent ral part
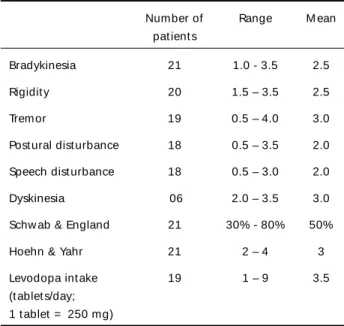
Table 1. Preoperat ive evaluat ion.
Number of Range M ean pat ient s
Bradykinesia 21 1.0 - 3.5 2.5
Rigidit y 20 1.5 – 3.5 2.5
Tremor 19 0.5 – 4.0 3.0
Post ural dist urbance 18 0.5 – 3.5 2.0 Speech dist urbance 18 0.5 – 3.0 2.0
Dyskinesia 06 2.0 – 3.5 3.0
Schw ab & England 21 30% - 80% 50%
Hoehn & Yahr 21 2 – 4 3
Levodopa int ake 19 1 – 9 3.5
and it s coordinat es are obt ained using t he resident soft -w are of t he M RI. The final coordinat es -w e use, t hough, are 2 mm lat eral t o t hose obt ained, in order t o reach prefe-rent ially t he lat eral t errit ory of STN.
St ill on T2-w eight ed coronal images, t he vert ical (Fig. 2A) and horizont al (Fig 2B) diamet ers of STN are measured, as w ell as t he dist ance of it s cent ral part from AC (number of slices post erior t o AC x slice t hickness = 1.5 mm) (Fig 3A and 3B), midline (lat eralit y) (Fig 4A) and apex of t he choroidal fissure (height ) (Fig 5B).
The M RI box is t hen replaced by t he CT (comput ed t omography) fiducials and a CT is performed (Somat on AR.C, Siemens) obt aining axial slices (t hickness and gap of 2mm) parallel t o t he frame base (all pat ient s underw ent CT). At t he end, a new t opogram is performed. Comparing t he angle of t he gant ry necessary t o obt ain slices parallel t o t he frame in bot h init ial and final t opograms, it is possi-ble t o verify if t here w as any movement during t he exam, in w hich case it is repeat ed. The coordinat es of AC, PC and M CP are derived from t he resident soft w are of t he CT. Usually, CT is performed just t o compare t he M CP (very close t o STN) coordinat es so obt ained w it h t hose derived from T1-w eight ed axial images. If t here is any ant eropos-t erior displacemeneropos-t of eropos-t he y coordinaeropos-t e oberopos-t ained from T1-w eight ed M RI, t he STN y coordinat e derived from T2-T1-w ei-ght ed coronal images can be correct ed.
Discrepancies bet w een M RI- and CT-derived coordi-nat es and t he final STN coordicoordi-nat es (place w here t he lesion is made) are calculat ed.
A sof t w are developed by t he Depart m ent of Neu-rophysiology of t he Universit y of Toront o is fed w it h t he CT-derived AC and PC coordinat es, w hich, in t urn, const ru-ct s a series of sagit t al diagrams ruled in a millimet er grid (based on digit ized plat es from t he Schalt enbrand and Wahren at las)32 st ret ched or shrunk t o mat ch t he pat ient ’s
int ercommissural (AC-PC) dist ance33. When only CT is used
t o est ablish t arget coordinat es, t hey can be easily read direct ly from t hese diagrams, usually 2-5 mm post erior, 2-4 mm below and 10-15 mm lat eral t o t he M CP33.
The same diagram33 is also used t o plan t he best t
rajec-tory to the target: to avoid “ contamination” of STN respon-ses t o st imulat ion w it h t hose from t he mot or t halamus, t he elect rode is commonly angled 40 t o 45° ant erior ly t o pass exact ly in front of t he last st ruct ure. The mediolat eral angle is usually zero.
The elect rode is driven t o t he t arget along t he planned t raject ory t hrough a 4 mm percut aneous t w ist drill hole placed just in front of t he coronal sut ure and about 13 mm lat eral t o t he midsagit t al plane (it depends on t he lat eral coordinat e of t he t arget ). It is 1.1 mm in diamet er w it h a 3-mm bare t ip (Diros Technology Inc., Toront o, Ca-nada). M acrost imulat ion is carried out in 2-mm st eps from
Fig 1. T2-w eight ed 3D coronal M RI. A- Landmarks f or ident if icat ion of STN (subt halamic nucleus): 14 t o 18 mm post erior t o AC (ant erior commissure); superior and medial t o t he apex of t he choroidal f issure; supe-rior and lat eral t o SNR (subst ant ia nigra pars ret iculat a); supesupe-rior, lat eral and ant esupe-rior t o RN (red nucleus); and an imaginary line passing t hrough t he lat eral border of t he brainst em usually crosses STN t hrough it s medial region; B- Habitual characteristics of the early-phase STN radiofrequency (RF) lesion: a three-concentric-zone image; C- Unusual charact erist ics of t he early-phase STN RF lesion: a hyperint ense image, probably correlat ed w it h a poor out come (only our t w o pat ient s harboring such a lesion present ed recurrence). Reprint ed under permission of Karger, Basel, f rom Vilela Filho O, et al.4.
B
6 mm above t he t arget t o 6 mm below it , st art ing, in each point , w it h a current of 0.5 Volt at 100 Hert z, w hich is increased in 0.5-Volt st eps t o a maximum of 3 Volt s. Aft er physiological det erminat ion of t he t arget (point w here st i-mulat ion produces t he best expect ed responses), a single lesion is made, under careful neurological monit oring: w e st art w it h 45°C/40 seconds and, if t here are no unt ow ard effect s, it is slow ly increased up t o 60-75°C/60 se conds, depending on t he result s obt ained. We use a radiofre-quency (RF) generat or/st imulat or for bot h st imulat ion and lesioning (OWL, model RFS-1, Diros Technology Inc., Toron-t o, Canada or M RFG-01B, M icromar, São Paulo, Brazil).
Post operat ive M RI, follow ing t he same prot ocol and slices’ orient at ion of t he preoperat ive M RI, is performed usually w it hin t he first t hree post operat ive days [(16
pa-t ienpa-t s); occasionally, for financial reasons, ipa-t is performed somet ime lat er (five pat ient s, from 13 t o 90 days aft er t he operat ion)]. Using T2-w eight ed coronal images, t he vert ical (Fig 2C) and horizont al (Fig 2D) diamet ers of t he lesion are measured, as w ell as t he dist ance of it s cent ral part from AC (Fig 3C and 3D), midline (Fig 4B) and apex of t he choroidal fissure (5-A). Comparing t hese dat a w it h t hose obt ained preoperat ively for t he STN, w e are able t o determine the territory of STN involved by the lesion, w hich w as classified as: lat eral, medial, mixed (lat eral and medial) and cent ral. In t he six pat ient s in w hom a preoperat ive M RI w as not performed and in t he pat ient in w hom STN could not be ident ified, t his det erminat ion w as based on t he comparison bet w een t he sit e and dimensions of t he lesion and of t he int act cont ralat eral STN.
Fig 2. Pre- and post operat ive M RI show ing, respect ively, t he vert ical (A) and horizont al (B) diamet ers of STN, and t he vert ical (C) and horizont al (D) diamet ers of t he lesion.
A C
Fig 4. Post - and preoperat ive T2-w eight ed 3D coronal M RI f rom a single pat ient show ing exact ly t he same height (6.1 mm) of t he lesion (A) and STN (B) in relat ion t o a horizont al line passing t hrough t he apex of t he choroidal f issure. Reprint ed under permission of Karger, Basel, f rom Vilela Filho O, et al.4.
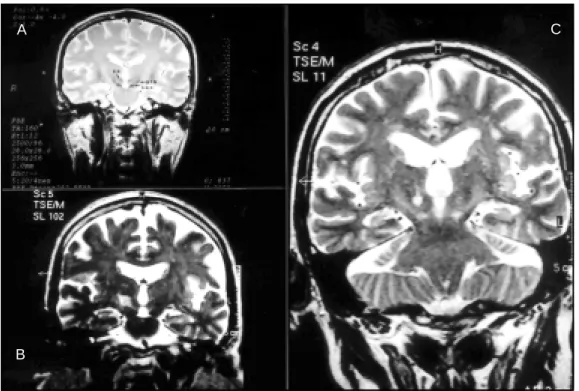
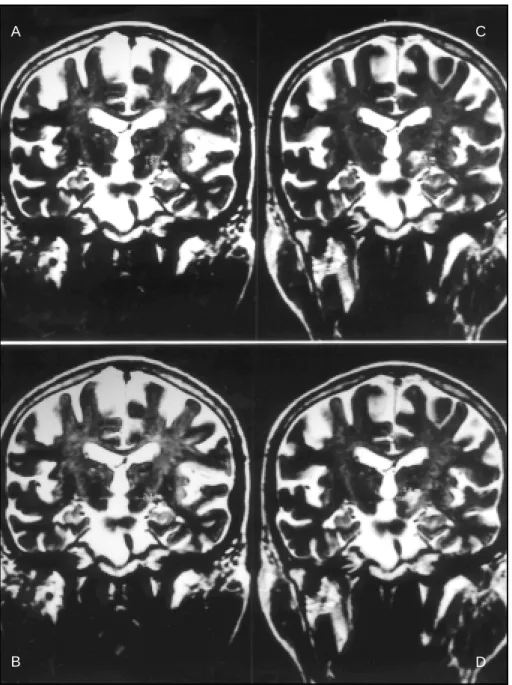
Fig 3. Pre- (upper row ) and post operat ive (low er row ) T2-w eight ed 3D coronal M RI f rom a single pat ient show ing exact ly t he same ant eropost erior dist ance (15 mm) bet w een STN [1 mm post (arrow in B)] or lesion [6 mm post (D)] and AC [14 mm ant (A) in t he preoperat ive image and 9 mm ant (C) in t he post operat ive image]. Reprint ed under permission of Karger, Basel, f rom Vilela Filho O, et al.4.,
A
C
B
D
A concomit ant Vim/VOp lesion (usually 7 mm ant erior t o PC and 2 mm above and 15 mm lat eral t o t he AC-PC line) w as performed in eight pat ient s (residual t remor, six pat ient s; surgery-induced dyskinesia, t w o pat ient s) w here macroelect rode st imulat ion produced t remor arrest (resi-dual t remor) or increased dyskinesia (ballism and chorea) w it h a current of 0.5-2 Volt s at 100 Hert z. The same lesio-ning paramet ers used for STN w ere used for Vim/VOp.
To have a more object ive and global evaluat ion of t he postoperative improvement, w e have created three indices, expressed in percent age: overall improvement index (OII), appendicular improvement index (AII) and midline impro-vement index (M II). The M II is obt ained by adding t he per-cent ages of speech and post ural dist urbances im pro-vement and dividing this sum by the number of these mani-fest at ions present ed by t he pat ient . The AII is expressed by dividing t he sum of t he percent ages of improvement of t remor, rigidit y, bradykinesia and levodopa-induced dys-kinesias by t he number of t hese manifest at ions present ed by t he pat ient . Finally, t he OII is obt ained by adding t he percent ages of improvement of t remor, rigidit y, bradyki-nesia, levodopa-induced dyskinesias, speech dist urbance, post ural dist urbance, S&E, H&Y and t he percent age of re-duct ion of levodopa int ake and dividing t his sum by t he number of t hese manifest at ions present ed by t he pat ient . The Wilcoxon t est w as used t o assess t he general im-pact of surgery on all recorded paramet ers. The M ann-Whit ney t est w as applied t o evaluat e t he significance of
sex, age, durat ion and st age (H&Y) of t he disease on t he OII, AII and M II and t o compare t he impact of t he different t errit ories of STN involved by t he lesion and t he cohort s w it h and w it hout a Vim/VOp lesion on all recorded para-met ers, including OII, AII and M II.
RESULTS
Parallelism bet w een t he f rame base and AC-PC line:
The orient at ion used for frame placement enabled us t o est ablish a parallelism bet w een t he frame and t he AC-PC line in 15 out of 21 pat ient s; in t he ot her six pat ient s, AC and PC appeared in adjacent axial slices.
Ident if icat ion and diamet ers of STN on T2-w eight ed 3D coronal images: STN ident ificat ion w as possible in 14 out of 15 p at ient s. It s m ean horizont al and vert ical diamet ers w ere, respect ively 6 mm (4-9.2mm) and 5 mm (3.4-6.4 mm).
Discrepancy bet w een CT and M RI-derived coordinat es and final STN coordinat es: The mean x, y and z coordinat e discrepancies w ere, respect ively: a. CT: 0.9 mm (0-3 mm), 0.6 mm (0-2 mm), and 1.6 mm (0-5 mm); b. M RI: 0.7 mm (0-2 mm), 0.9 mm (0-5.2 mm), and 1.1 mm (0-2.3 mm). It can be observed t hat M RI seems t o be more precise for t he acquisit ion of x and z coordinat es and CT, for t he y coordinat e.
Fig 5. Pre- and post operat ive T2-w eight ed 3D coronal M RI f rom a single pat ient show ing t he lat eralit y of STN [13 mm (A)] and lesion [13.8 mm (B)] in relat ion t o a vert ical line passing t hrough t he cent er of t he 3rd vent ricle. Not e t hat t he
lat eralit y of t he lesion is slight ly great er, in order t o involve t he lat eral region of STN, place of it s somat osensory t errit ory. Reprint ed under permission of Karger, Basel, f rom Vilela Filho O, et al.4.
Ef f ect s of macroelect rode st imulat ion and number of t racks:At t he t arget , st imulat ion produced pronounced al l evi at i o n o r el i m i n at i o n o f co n t r al at er al r i g i d i t y, bradykinesia and t remor in all pat ient s present ing t hese m anif est at ions and , in one out of six p at ient s w it h levodopa-induced dyskinesia, it produced dyskinesia.
One to five tracks w ere necessary for target confirmation (mean = 2.2); a single track was enough in 9 out of 21 patients.
STN coordinates in relation to the midcommissural point (MCP): The most prevalent coordinates w ere: 12.5 mm la-teral, 4 mm inferior and 3.5 mm posterior to the M CP.
Lesion diamet ers and aspect s on T2-w eight ed coronal images: The mean horizont al and vert ical diamet ers of t he lesion w ere, respect ively, 4.7 mm (2-8.7 mm) and 4.8 mm (2-7.5 mm).
According t o t heir aspect in t he post operat ive T2-w ei-ght ed images, t he lesions w ere classified as: hyperint ense (f our pat ient s) (Fig 1B), hypoint ense (one pat ient ; M RI p erf orm ed t hree m ont hs af t er t he p roced ure), t w o-concent ric-zone lesion (hyp oint ense inner zone and hyperint ense out er zone; five pat ient s), t hree-concent ric-zone lesion (hyperint ense inner ric-zone, hypoint ense middle zone and hyperint ense out er zone; t en pat ient s) (Fig 1C) and unseen (one pat ient ; M RI performed 61 days aft er t he operat ion).
The w hole core of hyper- and hypoint ense lesions w as considered w hen measuring t heir diamet ers. Wit h regard t o t he t w o- and t hree-concent ric-zone lesions, t he out er zone, considered by some as a mere edema34, w as not
included in t he measurement .
Territory of STN involved by the lesion: The STN territory in vo lved w as: lat eral (t h ree p at ien t s), m ed ial (f o u r
pat ient s), mixed (nine pat ient s) and cent ral (t w o pat ient s). It w as not possible t o est ablish t his relat ionship in t hree pat ient s: in one pat ient , t he lesion could not be seen in t he post operat ive M RI and in t w o pat ient s, t he est ablished prot ocol w as not obeyed in t he post operat ive M RI.
It is w ort h ment ioning t hat t he STN lesion ext ended t o SNR (subst ant ia nigra pars ret iculat a) in one pat ient , t o SNR and zona incert a in one pat ient and t o t he zona in-cert a in nine pat ient s; in ot her nine pat ient s, t he lesion did not ext end beyond t he limit s of STN.
Sex, age and durat ion of t he disease: There w as no st at ist ically signif icant dif f erence (M ann-Whit ney: α > 0.05) in clinical out come by sex. How ever, t here w as a non-st at ist ically significant (M ann-Whit ney t est : α > 0.05) t endency for bet t er result s in t hose pat ient s aged 60 years or less t han in t hose older t han 60 [£ 60 years (n = 11): OII = 61.4%, AII = 78.3% and M II = 64.1%; ≥ 60 years (n = 10): OII = 55.3%, AII = 77% and M II = 49.1%] and in t hose pat ient s present ing PD for six t o nine years (n = 5; OII = 67.7%, AII = 80.4% and M II = 77.6%) t han in t hose present ing it for less t han six years (n = 6; OII = 49.7%, AII = 68.7% and M II = 43.3%) or more t han nine years (n = 9; OII = 58%, AII = 81.2% and M II = 51.9%).
Impact of surgery: All recorded paramet ers w ere sig-nif icant ly im proved, as seen in Table 3. Som e point s, t hough, deserve furt her comment s.
In one pat ient w it h a 50% init ial relief of t he t remor, curiously, t he improvement cont inued and t he long-t erm follow up relief w as of 85.7%.
The H&Y improved by 0.5 in five pat ient s, 1 in eleven and 1.5 in one; four pat ient s remained unchanged. The pat ient s w it h H & Y = 3 or 3.5 (n = 10; OII = 63.8%, AII = 82.4% and M II = 67.5%) did bet t er t han t hose w it h H & Y
Table 2. M RI prot ocol.
T1-w eight ed T1-w eight ed T2-w eight ed spin-echo t urbo field echo 3D fast field echo 3D sagit t al sequence axial sequence coronal sequence
Field of view 260 mm 260 mm 260 mm
Rect angular field of view 100 mm 100 mm 100 mm
Repet it ion t ime 550 ms 20 ms 1500 ms
Echo t ime 16 ms 6.9 ms 50 ms
Turbo fact or ———— 30 ms 11 ms
Number of signal averages 2 1 2
M at rix size 256 x 256 256 x 256 256 x 256
Slice t hickness 6 mm 2 mm 1.5 mm
Int erslice gap 0.6 mm 0 mm 0 mm
< 3 (n = 6; OII = 54.2%, AII = 71.8% and M II = 60%) and H & Y = 4 (n = 5; OII = 52.9%, AII = 75.5% and M II = 34.3%); t his dif f erence, how ever, w as not st at ist ically significant (M ann-Whitney test: α > 0.05).
Due t o int olerance, t w o of our pat ient s w ere not using levodopa. The degree of reduct ion w as slight (33%) in t w o, signif icant (≥ 50%) in t hirt een and none in f our pat ient s. The pat ient s w ere able t o decrease levodopa int ake since t he early post operat ive phase.
Pat ient ’s self-evaluat ion: The mean self-report ed im-provement w as 84% (80-100%, 18 pat ient s; and 40-60%, 3 pat ient s).
Overall (OII), Appendicular (AII) and M idline (M II) Im p r o vem en t In d i ces: Th e OII, AII an d M II w er e, respect ively, 58.5%, 77.7% and 57.3%.
STN x Combined STN + Vim/VOp lesions: There w as
no st at ist ically signif icant dif f erence in any evaluat ed paramet ers bet w een t hese t w o cohort s (M ann-Whit ney t est : α > 0.05).
Lesioned STN t errit ory x Result s: The M ann-Whit ney t est show ed no st at ist ically significant difference bet w een t he lat eral and mixed subgroups (α > 0.05); how ever, t re-mor, OII and M II w ere bet t er improved by lat eral t han medial lesions (α < 0.05) and t remor, OII and S&E w ere bet t er improved by mixed t han medial lesions (α < 0.05). These findings suggest t hat lesioning of t he lat eral t errit ory (t he mixed lesion includes t he lat eral t errit ory) is impor-t animpor-t impor-t o achieve impor-t he besimpor-t resulimpor-t s.
Follow -up and Recurrence: All pat ient s w ere follow ed f or a m inim um of 9 m ont hs and a m axim um of 22.5 m ont hs (m ean f ollow -up of 13.5 m ont hs) by a single exam iner, a neurologist w it h exp ert ise in m ovem ent
disorders (DJS). Only six pat ient s w ere follow ed for less t han one year.
The recurrence rat e w as approxim at ely 10% (t w o pat ient s), and w as t aken int o account w hen w e calculat ed the percentage of improvement of all recorded parameters. It occurred w it hin t he first post operat ive mont h in bot h pat ient s, being only part ial for t remor and rigidit y, but complet e in one of t he cases of bradykinesia.
The ot her pat ient s have maint ained t heir improvement st able during t he w hole follow -up period.
Complicat ions: There w as no m ort alit y, inf ect ion, epilepsy or cognit ive impairment in our series.
Hemiballismus occurred in one pat ient , and mild t o moderate hemichorea, in another patient soon after the end of very successful operations. Both patients w ere treated w it h a Vim/VOp lesion performed in t he same surgical procedure, w ith immediate and complete resolution of their dyskinesias. So, in our series, t he incidence of surgery-induced dyskinesia w as approximately 10%.
There w as a 5% incidence of impairment of previous speech dist urbance (part ial recovery), t ransient (24 hours) impairment of previous post ural dist urbance, hypot onia (part ial recovery), and asympt omat ic int racerebral (fron-t al) hema(fron-t oma.
Transient confusion (less t han 48 hours) happened in eight pat ient s (38.1%): six of t hem w ere submit t ed t o STN lesioning (w it h ext ension t o t he zona incert a in t hree), and t w o underw ent STN + Vim/VOp lesioning. The medial t errit ory w as involved in seven of t he pat ient s in w hom t he lesion could be det ect ed by t he post operat ive M RI.
DISCUSSION
Why STN?
As ment ioned before, non-human primat e ani-mal models of M PTP-induced parkinsonism2
demons-Table 3. Impact of surgery.
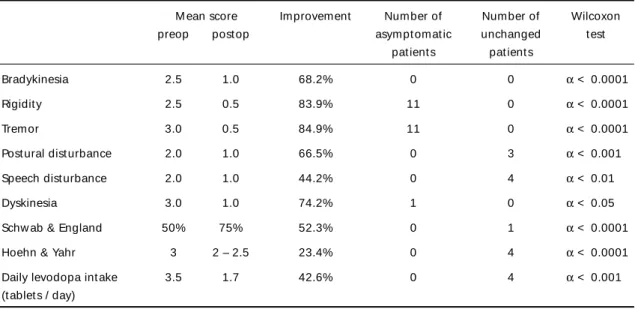
M ean score Improvement Number of Number of Wilcoxon
preop post op asympt omat ic unchanged t est
pat ient s pat ient s
Bradykinesia 2.5 1.0 68.2% 0 0 α < 0.0001
Rigidit y 2.5 0.5 83.9% 11 0 α < 0.0001
Tremor 3.0 0.5 84.9% 11 0 α < 0.0001
Post ural dist urbance 2.0 1.0 66.5% 0 3 α < 0.001
Speech dist urbance 2.0 1.0 44.2% 0 4 α < 0.01
Dyskinesia 3.0 1.0 74.2% 1 0 α < 0.05
Schw ab & England 50% 75% 52.3% 0 1 α < 0.0001
Hoehn & Yahr 3 2 – 2.5 23.4% 0 4 α < 0.0001
Daily levodopa int ake 3.5 1.7 42.6% 0 4 α < 0.001
t rat ed hyperact ivit y of t he STN and GPi. Such hype-ract ivit y induces hypoact ivit y of t he mot or t halamus and so, of t he mot or and pre-mot or cort ex, causing bradykinesia1,3,5-7. Hyperact ivit y of STN may also act
i-vat e t he nucleus t egm ent i pedunculopont is pars compact a (TPC), w hich, in t urn, excit es t he nucleus ret icularis gigant ocellularis, sit e of origin of t he ret i-culospinal t ract (RST). The RST is inhibit ory for t he I-B spinal cord int erneurons, w hich, in t urn, inhibit α -m ot or neurons1,3,6. It has been show n t hat t hose
int erneurons are hypoact ive in PD1,3,5,6. This
hypoac-t ivihypoac-t y m ay hypoac-t hen induce hyperachypoac-t ivihypoac-t y of α-m ot or neurons, t he pat hophysiological subst rat e of rigidit y, according t o one of t he accept ed hypot heses1,3,5,6.
Tremor cells have also been found in STN. Some au-t hors have even suggesau-t ed au-t haau-t au-t he Vim/VOp au-t re-mor cells represent not hing re-more t han t he t rans-mission of t he act ivit y of STN cells t o t he mot or t ha-lamus1,3,7,15,17. So, STN seems t o be involved in t he
genesis of t he main manifest at ions of PD. In fact , inact ivat ion of STN has a profound benefic effect on pract ically all manifest at ions of t he disease, bot h in humans and M PTP monkeys1,3,4,7,12-30,35,36. Another
im-port ant aspect is t he STN locat ion w it hin t he basal ganglia circuit ry: t hrough it s project ions t o t he GPi,
SNR and brainst em, it is in a unique posit ion t o in-fluence t he ent ire out put of t he basal ganglia1,3,5,7-10.
We have st at ed before t hat t he current know led-ge of basal ganglia funct ioning, w hich is based on M PTP monkey models and on t he subsequent obser-vat ions in humans, validat ed previously proposed cir-cuit s and physiology of t he basal ganglia. How ever, t here is one point deserving furt her comment s: con-sidering t he indirect basal ganglia circuit , t he STN w ould be hyperact ive in PD due t o hypoact ivit y of GPe (globus pallidus ext ernus). Nevert heless, it w as demonst rat ed experiment ally t hat t he GABA level at t he GPe-STN pat hw ay t erminals is normal and not diminished, as expect ed7. Besides, GPe lesioning
in-creases STN act ivit y only by 19.5%, w hile SNC lesi-oning increases it by 105.7%, suggest ing t hat STN hyperact ivit y w ould be secondary t o t he reduced le-vels of dopamine in t he t erminals of t he SNC-STN pat hw ay7. Int erest ingly, how ever, one of t he
resear-ches going on at one of t he aut hors (OVF) laborat ory is on t he effect of bilat eral st ereot act ic lesioning of t he GPe in int act Wist ar rat s (unpublished dat a): t he p r el i m i n ar y r esu l t s h ave co n si st en t l y sh o w n bradykinesia of such magnit ude as t o int erfere w it h Table 4. Improvement f ollow ing STN lesioning and STN-DBS (“ on” st at e).
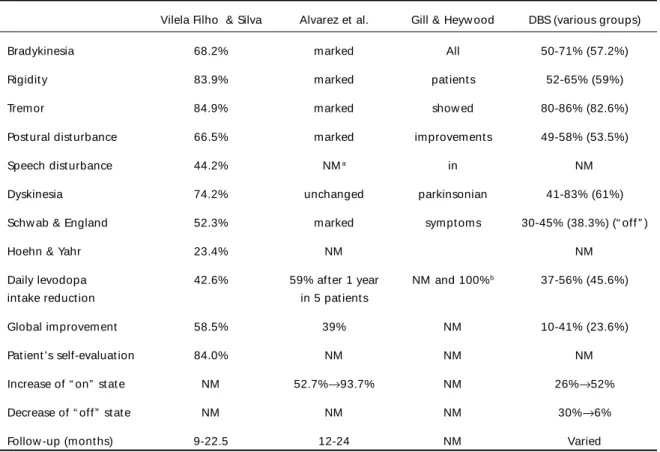
Vilela Filho & Silva Alvarez et al. Gill & Heyw ood DBS (various groups)
Bradykinesia 68.2% marked All 50-71% (57.2%)
Rigidit y 83.9% marked pat ient s 52-65% (59%)
Tremor 84.9% marked show ed 80-86% (82.6%)
Post ural dist urbance 66.5% marked improvement s 49-58% (53.5%)
Speech dist urbance 44.2% NMa in NM
Dyskinesia 74.2% unchanged parkinsonian 41-83% (61%)
Schw ab & England 52.3% marked sympt oms 30-45% (38.3%) (“ off ” )
Hoehn & Yahr 23.4% NM NM
Daily levodopa 42.6% 59% aft er 1 year NM and 100%b 37-56% (45.6%)
int ake reduct ion in 5 pat ient s
Global improvement 58.5% 39% NM 10-41% (23.6%)
Pat ient ’s self-evaluat ion 84.0% NM NM NM
Increase of “ on” st at e NM 52.7%→93.7% NM 26%→52%
Decrease of “ off ” st at e NM NM NM 30%→6%
Follow -up (mont hs) 9-22.5 12-24 NM Varied
t he animals’ feeding; some of t hem lost 50-100 g in five days. These findings, obviously, are complet ely opposit e t o t hose aforement ioned.
The STN present s four dist inct t errit ories7, w it h
different funct ions and connect ions: a-) t he dorso-lat eral sensorimot or t errit ory receives afferent s from GPe, mot or and premot or cort ex and project s t o GPe and put amen, being rich in kinest het ic and volunt ary cells and, in cases of PD, also of t remor cells; t he upper limb is represent ed in it s lat eral part and t he low er limb, in it s medial part ; b-) t he vent rolat eral associat ive t errit ory, w hich project s t o GPi, SNR and caudat um ; c-) t he vent rom edial lim bic t errit ory, w hich project s t o t he vent ral pallidum; and d-) t he dorsomedial oculomot or t errit ory. Considering t his anatomofunctional distribution, it is our opinion that the best STN region to be targeted is the lateral one. Furthermore, contrarily to Vim/VOp (the limits of GPi can be partly identified on IR M RI images), STN can almost alw ays be directly seen in M RI studies3,4,14,30,
w hich makes its targeting easier and independent of other landmarks commonly used in stereotactic sur-gery, such as AC and PC. Not less important, if STN hyperactivity really plays any role in the perpetuation of SNC dopaminergic cells death, its inactivation may have a protecting effect on PD progression3,4,7,11.
Vim/VOp t halamot omy or st imulat ion is usually indicated w hen the tremor is the most disabling mani-festation of PD1,31,37. How ever, even if bradykinesia is
not a significant feature by the time of surgery, w ith t he progression of t he disease it w ill event ually become, and then, even w ithout tremor, the patient w ill find his/her limb useless due to the bradykinesia.
GPi lesioning or st imulat ion is a very effect ive pro-cedure t o t reat bradykinesia, rigidit y and levodopa-induced dyskinesia1, 3, 31, 38. How ever, bot h procedures
are effect ive only w hile t he pat ient s are “ off ” , doing almost not hing w hen t hey are “ on” , except for t he elimination or alleviation of the dyskinesias1,3,20,38. The
levodopa int ake is also not affect ed by t hese proce-dures1,3,7,19. M any authors have claimed the successful
cont rol of t remor w it h t hese operat ions31, 38, 39. In our
hands, t hough, just mild t o moderat e t remor can be adequat ely cont rolled. Finally, t he visual response, a very import ant guide for t hese operat ions, is only very rarely elicit ed by st imulat ion. Even aft er iden-t ificaiden-t ion of iden-t he opiden-t ic iden-t raciden-t by microeleciden-t rode recor-ding, st imulat ion at t he same sit e st ill only rarely elicit s visual responses (Jerrold Vit ek, M D, personal communicat ion). Those w ho use macrost imulat ion as t he only means of physiological explorat ion, t hen, are at a great er risk t o lesion t he opt ic t ract , despit e
it s low incidence in t he majorit y of t he series, inclu-ding our ow n.
For all t hese reasons, STN seems t o be t he best t arget t o t reat PD.
St imulat ion or Lesioning?
But how t o best manipulat e t his t arget : by st i-mulat ion or lesioning? Act ually, t here seems t o be no doubt about it . M ainly due t o it s low er neuro-logical m orbidit y and reversibilit y, st im ulat ion is alw ays preferred t o lesioning. On t he ot her hand, t he former present s a number of mechanical com-plicat ions, a much higher incidence of infect ion (aro-und 8%), a high cost and t he necessit y t o change t he generat or every four t o seven years1, 7, 31, 33; such
short comings do not occur w hen lesioning is chosen. Anot her point t hat should be kept in mind is t he difficult y t o perform deep brain st imulat ion (DBS) in t hird w orld count ries, t he majorit y of t he count ries of t he w orld, due t o it s high cost . So, maybe t he best answ er t o t he init ial quest ion is t o t ry t o perform DBS in t he dominant brain hemisphere and lesioning in t he ot her one; if impossible, bilat eral lesioning may be cont emplat ed, but avoiding mirror lesions, w hich carry an increased risk of complicat ions. STN lesioning in one side and GPi or Vim/VOp lesioning (depending on t he main manifest at ions of t he dise-ase) in t he ot her side can be a reasonable solut ion. Finally, it is w ort h ment ioning t hat Gill & Heyw ood have performed bilat eral dorsolat eral STN lesioning w it hout significant side-effect s29,30.
Saf et y and Ef f icacy
The next import ant and inevit able quest ions are: is STN lesioning safe? How effect ive is t his procedure compared t o STN-DBS? The present w ork w as carried out in an at t empt t o answ er t hese quest ions. Since t he init ial report from Benabid et al. on STN-DBS in 199312,13, many groups7,14-25 have started similar trials,
reproducing t he excellent result s originally described by Benabid and his colleagues.
The result s of t hese groups7,14-25 show ed t hat
STN-DBS produced a mean improvement of t he UPDRS mot or scores of 53.8% (41-62%) in t he “ off ” st at e and of 23.6% (10-41%) in t he “ on” st at e. There w as a subst ant ial increase of t he “ on” period (from 26 t o 52%, in one st udy18) and a significant decrease of
t he “ off ” period (from 30 t o 6% in t he same st udy18).
Lesions of t he STN, mainly vascular in origin (he-m orrhages and inf arct s), have alw ays been asso-ciat ed w it h t he appearance of hemiballismus. For t his very reason, avoidance of STN lesioning has been considered a dogma in basal ganglia surgery. In fact , lesioning of STN is regarded as one of t he most con-sist ent animal models for movement disorders.
Aziz et al.7, Guridi et al.35, and Bergman et al.
(cit ed by t he previous aut hors7,35) perf orm ed STN
lesioning in M PTP monkeys obt aining marked im-provement of t he parkinsonian manifest at ions; sur-gery-induced dyskinesia (t ransient or permanent ), t hough, w as not an infrequent complicat ion. Limo-usin et al.23 t reat ed t hree parkinsonian pat ient s w it h
STN-DBS. One of t hem present ed hem iballism us, w hich disappeared by changing t he paramet ers of st im ulat ion. In anot her paper, Lim ousin et al.24
induced hemiballismus by STN-DBS in t w o parkin-sonian pat ient s in t he “ off ” st at e, w it hout previous levodopa-induced dyskinesia; t he paramet ers of st i-mulat ion t o produce ant i-parkinsonian effect s and hemiballismus, t hough, w ere complet ely different .
The aforement ioned experiment s show t hat STN lesioning may be a very effect ive procedure t o t reat PD, t hat dyskinesias are not a rule aft er t his proce-dure, despit e t heir significant incidence, and t hat STN-DBS may also produce dyskinesias.
Some report s on surgical t reat ment of PD using t arget s in t he subt halamic region (campot omy and subt halamot omy) show ed t hat t he dyskinesias are relat ively infrequent complicat ions, even w hen t he STN itself w as inadvertently lesioned28,35. Additionally,
ot her publicat ions demonst rat ed t he significant im-provement present ed by parkinsonian pat ient s w ho suffered from vascular lesions in t he STN28,40.
Considering all t hese findings, Obeso et al.26 first
report ed, in 1997, t he result s of STN lesioning in five pat ient s w it h PD.
Current ly, according t o a w ide review of t he lit e-rat ure1,3,4,7,14,17,26-30, t here are apparent ly only t hree
groups performing STN lesioning: one group, com-posed of t hree subgroups, led by Alvarez, Obeso and DeLong7,14,17,26-28; anot her group, led by Gill &
Hey-w ood29,30; and finally, our group, led by Vilela Filho
& Silva3,4.
Alvarez et al.27,28 report ed t he result s of unilat
e-ral STN lesioning in 11 pat ient s [t his series, appa-rent ly, compiles t he pat ient s previously report ed by Obeso et al.26 (five pat ient s), Rodriguez et al.17 (7
pa-t ienpa-t s) and Spa-t arr epa-t al.14 (one pat ient )]. CT w as used
t o obt ain t he t arget (dorsolat eral STN) coordinat es, commonly 2-3 mm post erior, 11-13 mm lat eral and 5-6 mm below t he M CP. Physiological explorat ion w as performed through semi-microrecording and sti-mulat ion. A 4-mm in diamet er radiofrequency lesion w as performed (60°C/30 seconds). All pat ient s un-derw ent an early post operat ive CT; only t hree pa-t ienpa-t s, how ever, underw enpa-t a pospa-t operapa-t ive M RI (11 mont hs aft er surgery), w hich show ed t he STN lesion ext ending 1-2 mm above. The aut hors did not men-t ion men-t he responses men-t o smen-t imulamen-t ion. A mean of 8.6 men-t ra-cks w as performed per pat ient . Dyskinesias occurred in t he cont ralat eral limbs during lesion-making in f ive pat ient s and subsided af t er 1-12 hours. Pos-t operaPos-t ive chorea of Pos-t he conPos-t ralaPos-t eral leg happened in one case and disappeared aft er five days. Anot her pat ient developed persist ent disabling cont ralat eral hemiballismus (st art ed on t he 7th post operat ive day),
w hich w as complet ely resolved by a medial palli-dot omy one year aft er t he first operat ion. No ot her complicat ions w ere ment ioned. A marked ameliora-t ion of parkinsonian feaameliora-t ures w as observed in all pa-t ienpa-t s pa-t he day afpa-t er surgery or even in pa-t he operapa-t ing room in t hose w it h t remor as a major manifest at ion. The follow -up varied from one t o t w o years. There w as a significant reduct ion in t he UPDRS mot or sco-res bot h in t he “ off ” (50%) and “ on” (39%) st at es; a similar effect w as observed for the UPDRS ADL scores. Both contralateral (mainly) and ipsilateral rigidity and bradykinesia w ere significant ly improved; t he ipsi-lat eral ameliorat ion, how ever, last ed just one year. Cont ralat eral t remor w as significant ly improved in all and eliminat ed in five out of eight pat ient s. M id-line manifest at ions w ere also significant ly amelio-rat ed. No recurrences w ere described. All pat ient s remained st able during t he w hole follow -up period.
Gill and Heyw ood30 performed a t ot al of 15 STN
movement s affect ing t he dist al low er limb, “ w hich t he pat ient did not not ice herself ” . There w ere no ot her side-effect s. Post operat ively, all pat ient s sho-w ed improvement s in parkinsonian sympt oms. In t heir first report29 of t w o pat ient s (included in t he
aforement ioned report , w e suppose) submit t ed t o bilat eral dorsolat eral STN lesion, t he UPDRS “ off ” mot or score improved from 50 t o 16 (68%) in one pat ient and from 64 t o 16 (75%) in t he ot her; bot h pat ient s w ere discharged w it hout levodopa and do-pamine agonist s present ing no not iceable “ off ” .
We performed a t rial of unilat eral STN lesioning in 23 pat ient s w it h PD. The last pat ient of t his init ial t rial w as operat ed in Sept ember 2000. Tw o pat ient s w ere excluded from analysis3, 4.
Fram e placem ent parallel t o t he inf raorbit al-ext ernal audit ory line enabled us, like St arr et al.14,
t o est ablish a parallelism bet w een t he frame and AC-PC line in t he majorit y of t he cases, w hich made t he calculat ion of t he t arget coordinat es easier and fas-t er. We w ere unable fas-t o idenfas-t ify fas-t he STN on T2-w ei-ght ed coronal images in only 1 out of 15 pat ient s; t he same happened t o St arr et al.14 in one out of six
pat ient s. In our hands, T2-w eight ed coronal images w ere at least as accurat e as CT axial slices t o obt ain STN coordinat es. Ot her aut hors, t arget ing ot her st ruct ures, have reached similar conclusions41.
Hut chison et al.15 also obt ained t remor arrest by
STN st imulat ion, but did not consider t his finding as an STN “ marker” , cont rarily t o Rodriguez et al.17.
Benabid’s group40 suggest ed t hat t he STN hallmark
t o st im ulat ion is t he d ecrease of w rist rigid it y. St imulat ion-induced dyskinesia is also t hought t o be a useful “ marker” of STN23,24,28. Unfortunately, Alvarez
et al.28 did not comment on t heir result s w it h STN
st imulat ion. Our experience w it h int raoperat ive STN st imulat ion show ed t hat t remor arrest (curiously, for some reason t hat w e fail t o recognize, t he lat ency f or STN st im ulat ion-ind uced t rem or arrest w as usually great er t han t hat observed for Vim st imula-t ion) and a pronounced decrease of imula-t he bradykinesia and rigidit y in bot h cont ralat eral limbs occurred in every pat ient harboring such manifest at ions. For t his reason, all t hese f indings should be regarded as hallmarks t o STN st imulat ion. St imulat ion-induced dyskinesia, t hough, happened in only one of our pat ient s, for w hich reason w e can not comment on it s value as an STN “ marker” .
Using macroelect rode st imulat ion, w e performed a mean of 2.2 t racks t o det ermine t he final STN coor-dinat es, w hich w ere usually 3.5 mm post erior, 4 mm inferior and 12.5 mm lat eral t o t he M CP, a bit post e-rior and supee-rior t o t hose report ed by Alvarez et al.28.
Post operat ive M RI, performed in all our pat ient s t o assess t he accuracy of lesion placement and it s aspect and dimensions, could not det ect any lesion in one of t hem (t he M RI could only be performed 61 days after the operation), despite the excellent results obtained. Likew ise, Krauss et al.34 performed late M RI
(usually six mont hs aft er t he operat ion) in 32 out of 36 pat ient s w ho underw ent medial pallidot omy; no lesion could be observed in t hree of t hem.
Using a met hod first described by our group, w e w ere able t o det ermine t he STN t errit ory involved by t he lesion. The best result s w ere obt ained w hen t he lat eral t errit ory w as involved, in keeping w it h t he know ledge t hat t he STN sensorimot or t errit ory is locat ed in it s dorsolat eral region and t hat t he STN efferent s t o GPi and SNR originat e from it s vent rola-t eral parrola-t7. Our point -of-view, t hen, differ a bit from
ot her aut hors28,30: w e t hink t hat bot h dorsolat eral
and vent rolat eral t errit ories should be lesioned t o achieve the best results and not only the dorsolateral territory. Alvarez et al.28, despite having performed
postoperative M RI in only 3 of their 11 patients, and Gill and Heyw ood30, despite the fact of not using any
measuring method to confirm their assumption, clai-med that the lesion w as placed in the dorsolateral STN.
The M RI aspect of a radiofrequency lesion w as first described by Tomlinson et al., cit ed by Krauss et al.34, in pat ient s submit t ed t o vent rolat eral t
halamo-t omy. Krauss ehalamo-t al.34 report ed a somew hat similar
result in pat ient s w ho underw ent medial pallidot o-my. The acute-phase aspect on T2-w eighted M RI ima-ges w as of a t hree-concent ric-zone lesion: a hyperin-t en se in n er zo n e, a h yp o in hyperin-t en se m id d le zo n e (t hought t o represent hemorrhagic coagulat ion ne-crosis) and a hyperint ense out er zone (t hought t o correspond t o edema), involved by addit ional peri-lesional edema, w it h a signal not as int ense as t he lesion out er zone; t he lat e-phase aspect w as of a hyperint ense lesion. We observed t w o ot her t ypes of acut e-phase lesions: hyperint ense and t w o-con-cent ric-zone (corresponding t o t he middle and out er zones of t he t hree-concent ric-zone lesion) lesions. Int erest ingly, t he t w o pat ient s w it h acut e hyperin-t ense lesions (fig. 1C) did nohyperin-t presenhyperin-t hyperin-t he same de-gree of improvement of t he ot her pat ient s. We also observed anot her t ype of lat e-phase lesion: hypoin-t ense lesion, seen in only one pahypoin-t ienhypoin-t w ho presenhypoin-t ed a very good result . The dimensions of our lesions w ere a lit t le bit larger t han t hose report ed by Alvarez et al.28 and Gill and Heyw ood29,30.
repor-t ed by Gill and Heyw ood (1 ourepor-t of 10 parepor-t ienrepor-t s30) and
by Alvarez et al. (1 out of 11 pat ient s w it h persist ent hemiballismus and 6 out of 11 patients w ith transient dyskinesia27, 28). Since bot h our pat ient s underw ent
Vim/VOp t halamot omy in t he same surgical proce-dure, achieving complet e and immediat e relief of t heir dyskinesias, w e do not know if t hey w ould be t ransient or persist ent .
It has been suggest ed28 t hat t he low incidence of
dyskinesia follow ing STN lesioning is due t o it s up-w ard ext ension t oup-w ards t he zona incert a, int errup-t ing errup-t he pallido-errup-t halamic paerrup-t hw ay, and errup-t hus mimi-cking t he effect of medial pallidot omy, w hich is w ell-know n t o abolish dyskinesias bot h in humans and monkeys. This hypot hesis is not support ed by our findings: t he STN lesion ext ended t o t he zona incer-t a in only incer-t en of our paincer-t ienincer-t s, including incer-t he paincer-t ienincer-t w ho developed hemichorea; on the other hand, none of t he nine pat ient s w it h lesions rest rict ed t o t he STN present ed dyskinesia (in t he pat ient w ho deve-loped hemiballismus, t he post operat ive M RI, unfor-t unaunfor-t ely performed only unfor-t w o monunfor-t hs afunfor-t er unfor-t he ope-rat ion, did not show eit her t he STN or t he Vim/VOp lesions). Anot her possibilit y is t hat t he t hreshold t o induce dyskinesia in pat ient s w it h PD is great er t han in normal individuals, w hich is in keeping w it h some experim ent al dat a show ing t hat dyskinesias are induced more frequent ly in normal t han in M PTP monkeys submit t ed t o kainic acid lesion of t he STN28.
The only ot her complicat ion t hat deserves some at t ent ion, due t o it s high incidence, is t he fort unat ely only t ransient confusion, present ed by eight of our pat ient s. In all cases (seven pat ient s) in w hich t he lesion could be ident ified by t he post operat ive M RI, t here w as at least a part ial involvement of t he medial region of the STN, w here its limbic territory is located. On t he ot her hand, seven ot her pat ient s w it h medial t errit ory involvement (medial and mixed lesions) did not present any confusion. In conclusion, all pat ient s w it h t ransient confusion present ed at least part ial medial territory involvement, but not all patients w ith m edial t errit ory involvem ent present ed t ransient confusion.
We have creat ed t hree new indices, t he overall improvement index (OII), t he appendicular improve-ment index (AII), and t he midline improveimprove-ment index (M II), w hich, in our opinion, provide a more object ive and precise assessment of t he pat ient ’s response t o t h e in st it u t ed t reat m en t t h an t h e o t h er scales current ly available3. The version here present ed is
t he first one, and it is our int ent ion t o bet t er elabo-rat e it in t he near fut ure.
In our series, all recorded paramet ers w ere signi-ficantly and greatly improved by the operation (Tables 3 and 4). Cont rarily t o Alvarez et al.28, t hough, t he
improvement w as observed soon after the procedure and only in t he opposit e hemibody and midline; re-duct ion of levodopa int ake w as possible since t he early post operat ive period; and t he levodopa-in-duced dyskinesias w ere subst ant ially relevodopa-in-duced. There w ere neit her st at ist ically signif icant d if f erences bet w een t he cohort s w it h and w it hout a Vim/VOp lesion nor sex-relat ed differences. Alt hough not st a-t isa-t ically significana-t , a-t here w as a sa-t rong a-t endency for pat ient s w ho w ere 60 or less years old, in st ages 3 and 3.5 of t he H&Y and w it h PD for six t o nine years t o present bet t er result s t han t he ot her pat ient s. The lat t er finding is part icularly int erest ing, since t here is no agreement in t he lit erat ure about t he ideal t ime t o indicat e surgery. Finally, our pat ient s self-report ed t heir improvement as a mean of 84%.
Cont rarily t o Alvarez et al27,28 and Gill and
Hey-w ood29,30, w ho did not ment ion any recurrence, w e
have observed a 10% recurrence rat e in our pat ient s, follow ed up for 9 t o 22.5 mont hs. Since t he early-phase post operat ive M RI show ed a hyperint ense le-sion (Fig 1C) in t he t w o pat ient s w it h recurrence, one could speculat e if t his finding may represent a poor prognost ic fact or.
Aziz presented orally his experience w ith STN lesio-ning for PD during the13th M eeting of the World
So-ciety for Stereotactic and Functional Neurosurgery, in 2001. Only a short-term relief of the manifestations of the disease w as observed in his series. The early-phase postoperative M RI aspect of the RF lesions pro-duced, how ever, w as of the hyperintense type and not of the expected three-concentric-zone type. This finding gives support to our hypothesis that the pre-sence of hyperintense lesions in the early postoperative M RI correlates w ith a poor surgical result and can be regarded as a poor prognostic factor.
In general, our result s compare favorably w it h t hose of STN-DBS and w it h t he t w o ot her series on STN lesioning (Table 4).
In conclusion, STN lesion is a very effect ive and safe operat ion, w it h a low recurrence rat e and an accept able complicat ion incidence. The most feared complicat ion, dyskinesia, can be successfully t reat ed in t he same surgical procedure or lat er by lesioning anot her t arget , Vim/VOp (in our series) or GPi (as report ed by Alvarez et al.28), w it hout increasing t he
Only after proving its safety and good and stable results in patients follow ed up for more than one year, w e restarted to perform STN lesioning. Since February 2001, 11 other patients have been operated, w ith re-sults somew hat similar to those already described.
In an attempt to avoid any misunderstanding w ith ot her operat ions previously performed in t he sub-t h alam ic reg io n , like cam p o sub-t o m y an d su b sub-t h a-lamot omy, w e w ould like t o suggest anot her t erm for t he relat ively new procedure here report ed: lat e-ral subt halamic nucleot omy.
Acknow ledgement s - The aut hors w ould like t o express t heir grat it ude t o t he colleagues Andy Parrent , Ron Tasker, Andres Lozano and Jerry Vit ek, for t heir advice, and t o Suzana Oellers, for proofreading t his manuscript .
REFERENCES
1. Vilela Filho O, Silva DJ. Doença de Parkinson: tratamento cirúrgico atual [Parkinson’s disease: current surgical treatment]. Neurocirurgia Contemporânea Brasileira 2000;3:1-8.
2. Parent A, Côté PY. Animal models of Parkinson’s disease. In Gildenberg PL, Tasker RR (Ed s.). Textbo o k o f stereo tactic and functio nal neurosurgery. New York: McGraw-Hill, 1998:1147-152.
3. Vilela Filho O, Silva DJ. Tratamento neurocirúrgico: lesão do núcleo subtalâmico [Neurosurgical treatment: subthalamic nucleus lesioning]. In Meneses MS, Teive HAG (Eds.). Doença de Parkinson [Parkinson’s disease). 2.Ed. Rio de Janeiro: Guanabara Koogan, 2002 (no prelo). 4. Vilela Filho O, Silva DJ, Souza HAO, et al. Stereotactic subthalamic
nucleus lesioning for the treatment of Parkinson’s disease. Stereotact Funct Neurosurg 2001;77:79-86.
5. DeLong MR, Vitek JL. Pathophysiological basis of neurosurgical treatment of Parkinson’s disease. In Gildenberg PL, Tasker RR (Eds.). Textbook of stereotactic and functional neurosurgery. New York: McGraw-Hill, 1998:1139-1146.
6. Mandir AS, Lenz FA. Clinical pathophysiology in Parkinson’s disease. In Gildenberg PL, Tasker RR (Eds.). Textbook of stereotactic and functional neurosurgery. New York: McGraw-Hill, 1998: 1133-1137. 7. Linazasoro G, Guridi J, Rodriguez MC, et al. Cirurgía del núcleo
subtalámico en la enfermedad de Parkinson [Surgery of the subthalamic nucleus in Parkinson’s disease]. Rev Neurol 2000;30:1066-1072. 8. Young AB, Penney JB. Biochemical and functional organization of the
basal ganglia. In Jankovic J, Tolosa E (Eds.). Parkinson’s disease and movement disorders. Baltimore: Williams & Wilkins, 1993:1-11. 9. A lexand er GE, DeLo ng MR, Strick PL. Parallel o rganizatio n o f
functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci, 1986;9:357-381.
10. Vilela Filho O, Souza JT. Síndrome de Tourette: anatomofisiologia dos gânglios da base e sistema límbico, circuitária e fisiopatologia [Tourette syndrome: anatomophysiology of the basal ganglia and limbic system, circuitry and pathophysiology]. In Santos MGP (ed). Síndrome de Gilles de la Tourette: tiques nervosos e transtornos de comportamento associados na infância e adolescência. São Paulo: Lemos Editorial, 1998: 137-157. 11. Rodriguez MC, Obeso JA, Olanow CW. Subthalamic nucleus-mediated
excitotoxicity in Parkinson’s disease: a target for neuroprotection. Ann Neurol 1998;44:S175-183.
12. Pollak P, Benabid AL, Gross C, et al. Effets de la stimulation du noyau sousthalamique dans la maladie de Parkinson. Rev Neurol (Paris) 1993; 149:175-176.
13. Benabid AL, Pollak P, Gross C, et al. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’ s disease. Stereotact Funct Neurosurg 1994;62:76-84.
14. Starr PA , Vitek JL, DeLo ng M, Bakay RA E. Magnetic reso nance imaging-based stereotactic localization of the globus pallidus and subthalamic nucleus. Neurosurgery 1999;44:303-314.
15. Hutchiso n W D, A llan RJ, O p itz H, et al. N euro p hy sio lo g ical identification of the subthalamic nucleus in surgery for Parkinson’s disease. Ann Neurol 1998;44:622-623.
16. Krack P, Benazzo uz A , Po llak P, et al. Treatment o f tremo r in Parkinson’s disease by subthalamic nucleus stimulation. Mov Disord 1998;13:907-914.
17. Rodriguez MC, Guridi OJ, Alvarez L, et al. The subthalamic nucleus and tremor in Parkinson’s disease. Mov Disord 1998;13 (Suppl 3):111-118. 18. Kumar R, Lozano A M, Kim YJ, et al. Double-blind evaluation of
subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Neurology 1998;51:850-855.
19. Krack P, Pollak P, Limousin P, et al. Subthalamic nucleus or internal pallidal stimulation in young onset Parkinson’s disease. Brain 1998; 121(Pt 3):451-457.
20. Kumar R, Lozano AM, Montgomery E, Lang AE. Pallidotomy and deep brain stimulation of the pallidum and subthalamic nucleus in advanced Parkinson’s disease. Mov Disord 1998;13(Suppl 1):73-82.
21. Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 1998; 339:1105-1111.
22. Benabid A L, Benazzouz A , Limousin P, et al. Dyskinesias and the subthalamic nucleus. Ann Neurol 2000;47:S189-192.
23. Limousin P, Pollak P, Benazzouz A, et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 1995;345:91-95.
24. Limousin P, Pollak P, Hoffmann D, Benazzouz A, Perret JE, Benabid AL. Abnormal involuntary movements induced by subthalamic nucleus stimulation in parkinsonian patients. Mov Disord 1996;11:231-235. 25. Limousin P, Pollak P, Benazzouz A, et al. Bilateral subthalamic nucleus
stimulation for severe Parkinson’s disease. Mov Disord 1995;10:672-674. 26. Obeso JA, Alvarez LM, Macias RJ, et al. Lesion of the subthalamic nucleus
(STN) in Parkinson’s disease (PD). Neurology 1997;48(Suppl 1):A138. 27. Alvarez L, Macias R, Guridi J, et al. Unilateral dorsal subthalamotomy
for Parkinson’s disease (PD). Mov Disord 1998;13(Suppl 2):266. 28. A lvarez L, Macias R, Guridi J, et al. Dorsal subthalamotomy for
Parkinson’s disease. Mov Disord 2001;16:72-78.
29. Gill SS, Heyw o o d P. Bilateral d o rso lateral subthalamo to my fo r advanced Parkinson’s disease. Lancet 1997;350:1224.
30. Gill SS, Heyw o o d P. Bilateral subthalamic nucleo to my can be accomplished safely. Mov Disord 1998;13(Suppl 2):201.
31. Gross RE, Lozano A M. The surgical management of Parkinson’ s disease. Contemporary Neurosurgery 1997;19:1-8.
32. Schaltenbrand G, Wahren W. Atlas for stereotaxy of the human brain. 2.Ed. Stuttgart: Georg Thieme, 1977.
33. Tasker RR, Vilela Filho O. Deep brain stimulation for the control of intractable pain. In Youmans JR (ed). Neurological surgery. 4.Ed. Philadelphia: W. B. Saunders, 1996:3512-3527.
34. Krauss JK, Desaloms JM, Lai EC, King DE, Jankovic J, Grossman RG. Microelectrode-guided posteroventral pallidotomy for treatment of Parkinso n’ s d isease: po sto perative magnetic reso nance imaging analysis. J Neurosurg 1997;87:358-367.
35. Guridi J, Herrero MT, Luquin R, Guillen J, Obeso JA. Subthalamotomy improves MPTP-induced parkinsonism in monkeys. Stereotact Funct Neurosurg 1994;62:98-102.
36. Guridi J, Herrero MT, Luquin MR, et al. Subthalamotomy in parkin-so nian mo nkeys. Behav io ural and bio chemical analysis: brain 1996;119(Pt 5):1717-1727.
37. Tasker RR. Thalamotomy. Neurosurg Clin N Am 1990;1:841-864. 38. Lozano AM, Lang AE. Pallidotomy for Parkinson’s disease.
Neurosur-gery Clin of N Am 1998;9:325-336.
39. Lozano AM, Lang AE, Hutchison WD. Pallidotomy for tremor. Mov Disord 1998;13(Suppl 3):107-110.
40. Benabid A L, Pollak P, Hoffmann D, et al. Chronic stimulation for Parkinson’s disease and other movement disorders. In Gildenberg PL, Tasker RR (Eds.). Textbook of stereotactic and functional neurosurgery. New York: McGraw-Hill, 1998:1199-1212.
![Fig 3. Pre- (upper row ) and post operat ive (low er row ) T2-w eight ed 3D coronal M RI f rom a single pat ient show ing exact ly t he same ant eropost erior dist ance (15 mm) bet w een STN [1 mm post (arrow in B)] or lesion [6 mm post (D)] and AC [14](https://thumb-eu.123doks.com/thumbv2/123dok_br/15429430.593772/5.892.180.757.685.1064/upper-operat-coronal-single-exact-eropost-erior-lesion.webp)
![Fig 5. Pre- and post operat ive T2-w eight ed 3D coronal M RI f rom a single pat ient show ing t he lat eralit y of STN [13 mm (A)] and lesion [13.8 mm (B)] in relat ion t o a vert ical line passing t hrough t he cent er of t he 3 rd vent ricle](https://thumb-eu.123doks.com/thumbv2/123dok_br/15429430.593772/6.892.107.744.96.515/operat-coronal-single-eralit-lesion-relat-passing-hrough.webp)