w w w . j c o l . o r g . b r
Journal
of
Coloproctology
Review
Article
Genetic
profile,
risk
factors
and
therapeutic
approach
of
desmoid
tumors
in
familial
adenomatous
polyposis
夽
Ana
Catarina
Ribeiro
Freitas
a,∗,
Laura
Elisabete
Ribeiro
Barbosa
a,baUniversidadedoPorto,FaculdadedeMedicina,Porto,Portugal
bHospitalSãoJoão,Servic¸odeCirurgiaGeral,Porto,Portugal
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received20December2016 Accepted2April2017
Keywords:
Desmoiddisease,hereditary Fibromatosis,aggressive Adenomatouspolyposiscoli Riskfactorsandtherapeutics
a
b
s
t
r
a
c
t
Introduction:DesmoidtumorsarethemainextraintestinalmanifestationofFAP,presenting highmorbidityandmortality.Itisaneoplasiawithoutmetastasiscapacity,butwith infiltra-tivegrowthandwithahighrateofrecurrence.Infamilialforms,thesetumorsareassociated withagerminalmutationintheAPCgene,withagenotype–phenotypecorrelation influ-encedbyotherriskfactors.
Materialsandmethods:A reviewofarticlespublished sincetheyear2000inPortuguese, English orSpanishondesmoidtumorsinpatientswithFAPwascarriedout.Atotalof 49publicationswereincluded.
Results:ThesiteofthemutationintheAPCgeneisrelatedtotheseverityofFAPandto thefrequencyofdesmoidtumor.Mutationslocateddistallytocodon1309areassociated withamoreattenuatedpolyposis,butwithhigherfrequencyofdesmoidtumors.Clinically, thesetumorsmayormaynotbesymptomatic,dependingontheirsizeandlocation.In theirtreatment,priorityshouldbegiventomedicaltherapy,especiallyinintra-abdominal tumors,withsurgerybeingthelastoptioniftherearenoothercomplications.
Discussion: Thesetumorsareassociatedwithcertainriskfactors:genetic(mutationsite), hormonal(estrogenicenvironment)andphysical(surgicaltrauma)ones.Inyoungwomen, alaterprophylacticcolectomyissuggested.Moreover,thelaparoscopicapproachto pro-phylacticsurgeryseemstobeanoptionthatreducessurgicaltraumaandconsequentlythe appearanceofdesmoidtumors.
Conclusion: Thestep-upmedicalapproachhasbeenshowntobevalidinthetreatmentof intra-abdominaldesmoidtumors,andmedicaltreatmentshouldbethefirsttherapeutic option.
©2017SociedadeBrasileiradeColoproctologia.PublishedbyElsevierEditoraLtda.This isanopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
夽
StudyconductedatUniversidadedoPorto,FaculdadedeMedicina,DepartamentodeCirurgiaGeral,Porto,Portugal.
∗ Correspondingauthor.
E-mail:ana.cat.freitas1@gmail.com(A.C.Freitas).
http://dx.doi.org/10.1016/j.jcol.2017.04.001
Perfil
genético,
fatores
de
risco
e
abordagem
terapêutica
dos
tumores
desmóides
na
Polipose
Adenomatosa
Familiar
Palavras-chave:
Doenc¸adesmoidehereditária Fibromatoseagressiva
Poliposeadenomatosadocólon Fatoresderiscoeterapêuticas
r
e
s
u
m
o
Introduc¸ão:Ostumoresdesmóidessãoaprincipalmanifestac¸ãoextraintestinaldaPAF, apre-sentandoelevadamorbimortalidade.Éumaneoplasiasemcapacidadedemetastizac¸ão,mas comcrescimentoinfiltrativoecomaltataxaderecorrência.Nasformasfamiliares associa-seaumamutac¸ãogerminativanogeneAPC,havendoumacorrelac¸ãogenótipo-fenótipo influenciadaporoutrosfatoresderisco.
Materiaisemétodos: Foiefetuadaumarevisãodeartigospublicadosdesdeoano2000,em português,inglêsouespanhol,acercadetumoresdesmóidesemdoentescomPAF.Foram incluídas,nototal,49publicac¸ões.
Resultados:Olocaldamutac¸ãonogeneAPCrelaciona-secomagravidadedaPAFefrequência detumordesmóide.Mutac¸õeslocalizadasdistalmenteaocodão1309associam-seauma poliposemaisatenuada,masamaiorfrequênciadetumordesmóide.Clinicamentepodem ser,ounão,sintomáticos,dependendodoseutamanhoelocalizac¸ão.Noseutratamento deveserdadaprioridadeàterapêuticamédica,sobretudonostumoresintra-abdominais, colocandoacirurgiacomoúltimaopc¸ão,casonãohajamoutrascomplicac¸ões.
Discussão: Estestumoresassociam-seadeterminadosfatoresderisco:genéticos(localda mutac¸ão),hormonais(ambienteestrogénico)e físicos(traumacirúrgico).Nasmulheres jovenssugere-searealizac¸ãodecolectomiaprofiláticamaistardiamente.Alémdisso, a abordagemlaparoscópicaparaacirurgiaprofiláticapareceserumaopc¸ãoquediminuio traumacirúrgicoeconsequentementeoaparecimentodetumoresdesmóides.
Conclusão: Aabordagemmédicaemstep-upmostrouserválidanotratamentodetumores desmóidesintra-abdominais,devendootratamentomédicoseraprimeiraopc¸ão terapêu-tica.
©2017SociedadeBrasileiradeColoproctologia.PublicadoporElsevierEditoraLtda.Este ´eumartigoOpenAccesssobumalicenc¸aCCBY-NC-ND(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
Introduction
Familialadenomatouspolyposis(FAP)isaninherited pathol-ogythatresultsfromtheautosomaldominanttransmissionof agermlinemutationintheadenomatouspolyposiscoli(APC) tumorsuppressorgenelocatedonthelongarmof chromo-some5.
Phenotypically,FAP ischaracterizedbythe development of hundreds to thousands of adenomatous polyps in the colonandrectum,withsubsequentriskofdeveloping colorec-talcarcinoma(CRC)ifaprophylacticproctocolectomyisnot performed.1
However, even after the proctocolectomy, patients with FAPpresenthighermortalityversusthegeneralpopulation, duetotheextra-colicmanifestationsofthisdisease:desmoid tumors,uppergastrointestinaltractadenomas,osteomas,and epidermoidcysts,aswellasthyroid,adrenalgland,and cen-tralnervoussystemneoplasms.2 Ofthese,desmoid tumors
arethemostfrequentcauseofdeathinpatientswithFAP.3
Desmoidtumorsdevelopfromconnectivetissue,fasciae, andaponeuroses,4,5correspondingtoamonoclonal
prolifera-tionofwell-differentiatedfibroblasts.6,7Fromthehistological
pointofview,thisisconsideredabenignneoplasm,sinceit does nothavemetastization capacity; but biologically,it is atumor withgreatlocal aggressiveness, giventhe infiltra-tivegrowthandtheinvasionofadjacentstructures,8besides
the high rate of local recurrence, even after its resection: 25–65%.2,5
Thisneoplasm mayoccur inthe contextofFAP or may arisesporadically;inthiscase,thetumorisaresultofsomatic mutationsintheAPCgeneorinthebeta-cateningene.9
Spo-radicdesmoidtumorsarerelativelyrare,affectingabout2–4 individualspermillioninthegeneralpopulation.10,11
As for the familial forms, the development of desmoid tumorsisrelated toagermlinemutationinthe APCgene, anditsfrequencyismuchhigher.Infact,itisestimatedthat between10and25%ofpatientswithFAPwilldevelopatleast onedesmoidtumorthroughouttheirlives,12,13witharisk850
timeshigherthanthatforthegeneralpopulation.5
Regardingtheriskfactorsforthedevelopmentofdesmoid tumors ina FAP context, studies point toseveral possibil-ities, such as family history, previous abdominal surgery, and gender, inadditionto thesite ofmutationinthe APC gene,10,14,15 which is also related to the severity of these
tumors.16
Materials
and
methods
AliteraturesearchthroughtheMEDLINEdatabaseviaPubMed was performed, and different combinations of terms were used: desmoid disease, hereditary; familial adenomatous polyposis; fibromatosis, familial infiltrative; adenomatous polyposiscoli;riskfactorsandtherapeutics.
Inthisstudy,theinclusioncriteriawerepublicationsfrom theyear2000andtextinPortuguese,EnglishorSpanish.The titlesandabstractswereread,withprioritygiventothemost recentand completearticles thatcorrespondedtothe pro-posedobjectives.Subsequently,otherarticleswereidentified bycross-referencesofarticlesobtainedintheinitialsurvey.In total,49publicationswereincludedinthisstudy.
Results
ThedevelopmentofdesmoidtumorsinpatientswithFAPis describedinseveralstudiesasaconditionassociatedwithrisk factors,namely,apositivefamilyhistory,previousabdominal surgery,and,morerecently,thesiteofthemutationintheAPC gene.17
Infact,thegenotype–phenotyperelationshiphasbeen ana-lyzedinseveralstudies,and theirconclusionsaremuchin thesameline:thosemutationslocateddistallytocodon1309 andespeciallyatthe3′ endareassociatedwithahighrisk
ofdevelopingdesmoid tumors,andparticularlyinasevere form.10,18,19
AccordingtothestudybyBertarioetal.,patientswithFAP presenta12-foldhigherriskofadesmoid tumorwhenthe mutationislocatedbeyondcodon1444(“desmoidregion”) ver-suspatientswhosemutationsarelocatedmoreproximally.12
Inaddition, it hasfurtherbeen shownthat theseverity ofthedisease andofthetumormaydependonthesiteof themutation. JeanCouture et al.20 showedthat mutations
atcodon1309areassociatedwithasevereformofFAP,with thedevelopmentofpolypsandCRCatanearlierage, com-paredtomutationsbetweencodons1445and1578,whichare associatedwithamoreattenuatedpolyposis,butwithgreater frequencyofdesmoidtumors.20
Ontheotherhand,currentlythefunctionoftheAPCgeneis onlypartiallyknown;therefore,onecannotmaintainthatthe moredistalthemutation,thegreatertheriskofoccurrenceof adesmoidtumor.Todate,morethan400germlinemutations havebeendescribedinthistumorsuppressorgene,mostof whichresultinatruncatedprotein.
Experimentalinformationhasshownthatmutationsinthe APCgenehave thepotentialto causelossof beta-catenin-mediatedregulationofsignaltransductionpathways.12
ThesegmentoftheAPCgenebetweencodons1265and 2035containsthe nucleotidesthat encodethe APCprotein bindingzone tobeta-catenin,thebindingofwhichtriggers beta-catenindegradation(whichfunctionsasatranscription factor). Thus, the integrity ofthe APC proteinis essential, because it was found that the presence of binding sites alteredbymutationsinthereferredsegmentcompromisesthe degradationofthebeta-catenintranscriptionfactor,affecting
celldifferentiation, proliferation,andapoptosis,which con-tributestothemanifestationsofFAP.10,21
Dependingontheirsizeandlocation,thedesmoidtumors may beclinicallyasymptomaticor maypresent manifesta-tions,usuallywithcompressivesymptoms.Inmanypatients, intra-abdominaltumorsareasymptomatic,appearingas acci-dental findings on the occasion of a physical or imaging examination. Whenthese tumorsreach largerdimensions, theycancause intestinalobstruction,mesentericischemia, hydronephrosis,fistulization,andsepsis,amongothers.4,22
Bothcomputedtomography(CT)andmagneticresonance imaging (MRI) can be used for the detection of desmoid tumors.8However,theconfirmationdiagnosisisestablished
byhistologyandbyimmunohistochemistry.23,24CThasbeen
consideredasthegoldstandardamongimagingmethodsin theassessmentofresponsetotreatment.Fortumorsofsmall size(<2cm)or forthe follow-up ofyoung patientswithan interestinavoidingtheradiationeffect,MRImayreplaceCT asafollow-upmethod,andmayalsobeusedtobetter trans-latethetumoraggressiveness,bydemonstratingthedegreeof cellularityandvascularization.22
Thereisstillnoprotocolforthetreatmentofthesetumors, duetotheirprevalenceandlackofknowledgeoftheir etiol-ogyandbiology.4,25 For themostpart,the desmoidtumors
growslowly;buttherearesituationsinwhichsuchtumors growrapidly,beingrefractorytoanytreatment.26Inaddition,
although they do not metastasize, their malignant poten-tialcomesmainlyfromtheirinfiltrativepowerandrelapsing nature.
Althoughsurgeryhas beenthe maintherapeuticoption withcurativepotential,itsuseiscontroversial,sinceit rep-resentsoneoftheriskfactorsfortheonsetandrecurrenceof thesetumors,sincetherecurrencerateisveryhigh,evenin thoseresectionswithfreemargins.10Thus,giventhelackofan
idealtreatment,thetherapeuticdecisionbecomesatrue clin-icalchallenge,oftenrequiringamultidisciplinaryapproach.
Currently,theproposedtherapiescanbedividedintofour different groups: pharmacological treatment, radiotherapy, chemotherapy,andsurgery.22
Oneofthepossibilitiesofmedicaltreatmentistheuseof non-steroidalanti-inflammatorydrugs(NSAIDs),sinceseveral studieshaveshownanefficacyratecloseto50%inreducing desmoidtumors.1,8 Currently,sulindacandcelecoxibarethe
pharmacologicalagentsused. Studiessuggest thatthe first choiceformedicaltreatmentshouldbeoneoftheNSAIDsand, inthecaseoffailure,theoptionwouldbeacombinationof NSAIDswithhormonaltreatment.1,8,27
In fact, hormonal agents are another proposed therapy for thesetumors, consideringthat the higher incidencein females,theassociationwithoralcontraceptiveuse,andthe highergrowthoftumorsinpregnantwomenseemtospeak in favor of the role of estrogens In their appearance and growth.28–31Inaddition,thefactthatthistypeof
fibromato-sisismorecommoninwomenofchildbearingage,regressing withmenopauseorafteroophorectomy,servestoconfirmthis relationship.29,31
Tamoxifenandtoremifenearetheantiestrogenagentsthat havebeenmorewidelyused.1,27,29AccordingtoBocaleetal.,29
ofoneoftheseagentsdoesnotimplyfailurewiththe oth-ers.Accordingtotheavailabledata,theefficacyoftamoxifen isthesameasfortoremifene;However,insomecases,this latteragentappearstobeeffective assecond linetherapy, afterthe occurrenceof resistancetotamoxifen. Thestudy also shows that antiestrogen therapy works on both FAP-associatedtumorsandonsporadictumorsand,similarly,on primaryorrecurrenttumors.29
Clinically,thishormonetherapydoesnotappeartohave relevantsideeffects,includinginmen;thus,thisoptioncanbe used.Inwomenwithlong-termtreatment,frequentchanges intheirmenstrualcycleareobserved;insuchcases, endovagi-nalultrasoundisrecommended.29
Thisandotherstudiesalsoemphasizethefactthat tamox-ifenandotherantiestrogenagentsproducearesponse,even inestrogenreceptor-freedesmoidtumors.29,30,32
Thus,it is proposed that, inrecurrent desmoid tumors, preferenceshouldbegiventotamoxifentherapy,ratherthan radiotherapyor surgicalre-exploration. Especiallyinyoung women,tamoxifenshouldbeconsideredastheinitialchoice becausethisagentcontributestoagreaterpossibilityof pre-servingtheirfertility.30
Allinall,themedicaltreatmentencompassestheuseof NSAIDsaloneorincombinationwithhormonalagents,aswell asthemonotherapywiththeselatteragents.
Some studies have compared treatments for desmoid tumors (associated with FAP, or sporadic ones) and have reachedtoasignificantlyhigherpercentageoftotalorpartial remissionwiththe exclusiveuse ofhormonalagents com-paredtotheirassociation withNSAIDs.Itappearsthatthe NSAID+anti-estrogen combination didnot addany benefit tothetreatment,althoughtherehavebeenotherreportsof successfulcaseswith the use ofNSAIDasmonotherapy.29
Thisallowsustoconcludethattherearestilluncertainties regarding the biological and therapeutic behavior of these tumors.
Currently,combined therapiesare usedmainly incases ofinoperabletumorsand alsoinsituationsofpost-surgical recurrencesandintheperioperativeperiod.33However,this
may be a valid option for intra-abdominal tumors, which wouldallowavoidingsurgicaltrauma.
Anotheralternativetherapytosurgeryisradiationtherapy. ThestudybyNuyttensetal.,involvingcasestreatedonlywith surgery,withsurgeryassociatedwithradiotherapy,andonly withradiotherapy,demonstratedacontrolrateof61%,78%, and75%,respectively.34
In addition, Turina et al.35 suggest a good control
of large unresectable desmoid tumors with radiother-apy/brachytherapy.Brachytherapyisanotherproposedoption; foritsuse,thetumorsmustbeofanadequatesize,inorderto becorrectlyexposedtotheapplicators.35
Althoughthereissomecontroversy,thereisevidencethat radiotherapy can model fibrosis, with increasing evidence favoringadecreaseinthepercentageoflocalrecurrencewith theuseofthisoption,bothbeforeandaftersurgery.1,24
Studiesalsoconfirmtheimportantrolethatradiotherapy canplayinthetreatmentofunresectabletumorswith extra-abdominallocationandintheabdominalwall,aswellasinthe post-surgicaltreatmentofcaseswithpositivemargins.8 But
intra-abdominaltumorsappeartohavelowerradiosensitivity,
andthismakesradiotherapyrarelyusedintumorswiththis location;besides,isalsotheriskofaradiationenteritis.1
Anothertherapeuticoptionischemotherapy.Lowdosesof doxorubicinincombinationwithdacarbazine,aswellasthe combinationofmethotrexate+vinblastine,alsoatlowdoses, havedemonstratedefficacyinthetreatmentoftumorswith progressivegrowthandnoresponsetomedicaltherapy.8,27,36
OneoftheexamplesisdescribedinthestudybyToiyama et al.,37 which shows the efficacy of a low-dose
combina-tionregimenofmethotrexateandvinblastineina26-year-old patientwithamesentericdesmoidtumor.Thepatientwas treatedwiththeabove-mentionedregimentwiceaweekfor12 months,andtheauthorsnotedadecreaseintumorsizewith subsequentregressionandwithnosignificantsideeffects.37
Therefore, chemotherapy may be considered as a ther-apeutic option in tumors that show a progression after othertherapies.Regardingthepossibletherapeuticregimens, thecombinationsdoxorubicin+dacarbazineand methotrex-ate+vinblastinehavebeenusedmoreoften;butother effec-tivecombinations,suchascyclophosphamide+doxorubicin, oreventhecombinationofdoxorubicinwiththemonoclonal antibodyimatinib,havebeendescribed.35Itseemsthatthe
latteragentmayalsoplayanimportantroleinthetreatment ofthesetumors.38
Finally,withrespecttosurgery:untilnow,thisisthe ther-apeutic standard,althoughmuchoftheliteraturedoesnot recommendthesurgicalsolutionasthefirstlineoftreatment, especially in patients with intra-abdominal tumors.2,5 For
tumorswiththislocation,thelowrateofcompleteresection and, consequently,the high percentageofrecurrences and morbidity, makesurgeryan optionthat isincreasingly rel-egated to the end of the line of treatments. Due to the potentialmorbidityandmortality,forexample,hemorrhage, shortbowelsyndrome,orevenpostoperativedeath,studies suggest that surgeryshouldonlybeconsidered inpatients withdesmoidtumorsresistanttomedicaltreatmentorin pal-liativesituations,inordertocontrolthesymptomscausedby theirprogression,suchasintestinalocclusion,ischemiaand enteric fistulization,and hydronephrosis,amongothers.4,39
Ontheotherhand,intumorsoftheabdominalwallorwith anextra-abdominallocation,surgerymaybeanappropriate treatment.40
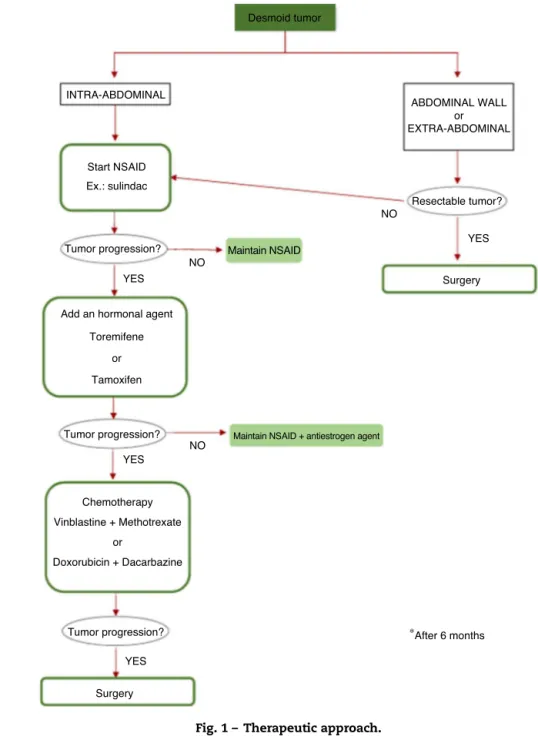
Thus, intra-abdominal desmoid tumors should be approached according to a step-up strategy, that is, with the progression of the aggressiveness of the treatment in function of the evolution of the disease.22 According to
Latchford et al.,1 the treatment ofintra-abdominaltumors
shouldbestartedwithsulindac(150mg);ifthistreatmentis noteffective, ananti-hormonalagentshouldbecombined: toremifene (180mg) or tamoxifen (120mg). Having tumor progression, chemotherapyshould be the next choice(low doseofvinblastine+methotrexate).Therefore,surgeryshould bereservedforseverecomplications.1
Discussion
overexpressioninthenuclei;ontheotherhand,incasesof FAP,agermlinemutationispresentinthedesmoidregionof theAPCgene.41
Thisis a groupof heterogeneoustumors, ranging from smallmesentericplaquestodesmoidtumorsthatoccupythe entireabdominalcavity,andmaystillpresentthemselveswith alittleaggressivecharacteror,onthecontrary,withgreatlocal aggressivenessandarapidinfiltrativegrowth.
Histologically, the tumor is composed mainly of highly differentiatedfibroblastswithanelongatednucleus; conse-quently,theyrarelymetastasize.33InpatientswithFAP,this
typeoftumortendstoarisemainlyinthemesenteryofthe smallintestine,followed,inorderofprevalence,bythe abdom-inalwallandfinally,theextremitiesandthetrunk.Theselatter regionsarerarelocations,inacontextofFAP.10
Clinically,thesetumorsrepresentthemostrelevant extra-colonicmanifestationofFAP,giventheassociatedmorbidity andmortality(secondcauseofdeath,shortlyafterCRC). Eti-ologically,wedonotyethaveatheorytoexplaintheorigin ofthesetumors;however,ithasbeenshownthatmutations in the APC gene predispose to the development of fibro-matosis(geneticfactor),aswellastohormonalandphysical factors.42Regardingthelatter,itisverifiedthat,afterthe
sur-gicalintervention,adisproportionateinflammatoryresponse tothetraumaisnoted,consequentlywithanaccumulation offibroblasts.33Thereisevidenceimplicatingsurgicaltrauma
asoneofthefactorscapableofleadingtothedevelopment ofthistypeoftumor,andthereisnocorrespondencewitha specifictypeofsurgery.Thefrequencyofdesmoidtumorsin patientsundergoingproctocolectomyproceduresissimilarto thatinpatientstreatedbypartialcolectomyorbycolectomy withileorectalanastomosis.12
Infact,inpatientswithFAPtreatedwithprophylactic colec-tomy,themortalitycomesmainlyfromthedesmoidtumors. During the first 5 years after surgery, one in six patients developsadesmoidtumor,usuallywithanintra-abdominal location.
Inthissense,Vitellaroetal.43investigatedwhether
laparo-scopicsurgerywasassociatedwithalowertumorriskwhen comparedtoopensurgery.Withthetwostudygroups show-ingsimilarcharacteristicsbeforesurgery,ina5-yearfollow-up period,the authorsobservedalower incidenceofdesmoid tumorsinthegroupundergoinglaparoscopicsurgery. How-ever, given the reduced sizeof this series and the limited follow-up period, this study points only to the possibility thatlaparoscopicsurgeryreducedtheriskofoccurrenceofa desmoidtumorafterprophylacticcolectomyinpatientswith FAP.43
Another study also shows that in cases of prophylac-ticsurgerywithanileorectalanastomosis,the laparoscopic approachresultsinfewercomplicationsandfasterrecovery.44
Thus,itisconcludedthatlaparoscopicsurgerymayreduce theriskofthesetumors;however,furtherstudiesareneeded, sothatthistypeofsurgerymaybetherecommended proce-dure.
Regardingthegeneticfactor,ithasalreadybeenshownthat mutationsinthe APCgene are apparently thesingle most importantriskfactorfortheonsetofthedesmoidtumorin patientswithFAP.12 AccordingtoSpeakeetal.45 85%ofthe
mutationsintheAPCgenearelocatedbetweencodons1250
and1464,andcorrespondencewasfoundbetweenthe muta-tionsiteandFAPseverityandfrequencyofdesmoidtumors, whichagreeswithfindingsinotherstudies.Insituationsof anattenuatedFAP,mainlyassociatedwithmutationsatthe 3′ end, agreatertendencyisobservedfordesmoidtumors.
Considering this inverse relationship, the study concludes that,inpatientswithmutationsinthislocation,abdominal surgeryrepresentsagreatriskforthedevelopmentofdesmoid tumors,proposingthat,incasesofattenuatedFAP,thebest prophylacticstrategywouldbeanendoscopicsurveillanceof polyps.Incasesofpatientswiththismutation,butwhoshow signsofprogressionofthepolyps,evenafteranattemptof endoscopiccontrol,orinthefaceofthepresenceofa carci-noma,surgerybecomesessential.45However,itisadvisable
toadoptchemoprophylaxis(withsulindacortamoxifen),in ordertotrytocontroltheriskofdevelopingadesmoidtumor, thusimprovingthepost-surgicalprognosis.46
Despiteadvancesingeneticknowledgeandsurgical tech-niques,thetypeandtimingofaprophylacticsurgeryremain uncertain. Ontheonehand,thesoonerthe surgeryis per-formed, thegreater the chancesofCRCprevention;on the other hand, some authors have suggested that, in young women, prophylacticcolectomyshouldbeconsideredlater, sincethesepatientsareatahigherriskforthedevelopment ofdesmoid tumorsifthesurgeryisperformedatanearlier
age.43,47,48
Moreover,ithasbeendemonstratedthat, incomparison to male patients, women are atincreasedrisk of develop-ing tumorsafter surgery – afact which provesthat in an estrogenic environment, surgical trauma is a predisposing factorfortheonsetofthesetumorsinfemales.10,12 Infact,
the developmentof desmoid tumorsbefore the surgery is morefrequentinmale patients.Thisfindingsuggeststhat, inmales,thesiteofthemutationinAPCgeneisthefactor withthegreatestinfluenceontheappearanceofthetumor, with alower correlationwith surgicaltrauma.12 Thestudy
alsoshowsthatinthefemale groupwithFAPthedesmoid tumorsweremorefrequent,butthatthemalepatients,when affected,tendedtopresenttumorswithmoreaggressive char-acteristics,ofgreater dimensionsand withmorefrequency oflocationintheabdominalwall,comparedwiththefemale group.12
Therefore,therearesignificantgenderdifferencesinthe manifestation of desmoid tumors. Female patients are at higherriskoftumoroccurrence,regardlessofthesiteofthe mutation,whileinthemalegenderthesiteofthemutation seemstoexertagreaterinfluence.Thereasonsforthese dif-ferencesmaybeexplainedbythedifferentimpactoftherisk factors:surgicaltrauma,hormonalinfluence,andlocationof themutation.
Desmoid tumor
INTRA-ABDOMINAL
Tumor progression?
Tumor progression?
Tumor progression?
YES
Surgery
Surgery ABDOMINAL WALL
or EXTRA-ABDOMINAL
Resectable tumor?
∗After 6 months
Chemotherapy Vinblastine + Methotrexate
or
Doxorubicin + Dacarbazine
Maintain NSAID + antiestrogen agent
YES
YES
YES Add an hormonal agent
Toremifene
or
Tamoxifen
NO
NO
NO
Maintain NSAID Start NSAID
Ex.: sulindac
Fig.1–Therapeuticapproach.
tumorprogression,chemotherapyshouldbestarted.Thereby, surgerywillbethelastoption(Fig.1).
Conclusion
FAP-associated desmoid tumors arise after a prophylactic colectomyandaffectmainlyfemalepatients.However,when thesetumorsariseinmen,theytendtobemoresevere,not havingadirectrelationtothesurgicalintervention.
Mutationsatthe 3′ endofthe APCgene,and especially
mutationsbeyondcodon1444,arephenotypicallycorrelated withlesspolyposis (attenuatedFAP),butalsowithahigher riskofdeveloping thetumor. Sincethe surgicalaggression inherent to a procedure of prophylactic colectomy is an
important risk factor for the development of this type of tumor,itisquestionedwhethertheendoscopicsurveillance ofpolypsisanadequatemeasureasaprophylacticstrategy forcoliccarcinoma.Incasesinwhichsuchameasureisnot possible,thelaparoscopicapproachseemstopresentbetter preventiveresults.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. LatchfordAR,SturtNJ,NealeK,RogersPA,PhillipsRK.A 10-yearreviewofsurgeryfordesmoiddiseaseassociatedwith familialadenomatouspolyposis.BrJSurg.2006;93:1258–64.
2. RighettiAE,JacominiC,ParraRS,AlmeidaAL,RochaJJ,Feres O.Familialadenomatouspolyposisanddesmoidtumors. Clinics.2011;66:1839–42.
3. Seow-ChoenF.Themanagementofdesmoidsinpatients withfamilialadenomatouspolyposis(FAP).ActaChirIugosl. 2008;55:83–7.
4. TanakaK,ToiyamaY,OkugawaY,HiroJ,KawamotoA,Inoue Y,etal.Cytoreductivestrategyformultipleintra-abdominal andabdominalwalldesmoidtumorsinfamilial
adenomatouspolyposis:reportofthreecases.ClinJ Gastroenterol.2012;5:361–6.
5. FerencT,SygutJ,Kopczy ´nskiJ,MayerM,Latos-BielenskaA, DzikiA,etal.Aggressivefibromatosis(desmoidtumors): definition,occurrence,pathology,diagnosticproblems, clinicalbehavior,geneticbackground.PolJPathol. 2006;57:5–15.
6. ColomboC,FooWC,WhitingD,YoungED,LusbyK,Pollock RE,etal.FAP-relateddesmoidtumors:aseriesof44patients evaluatedinacancerreferralcenter.HistolHistopathol. 2012;27:641–9.
7. FioreM,RimareixF,MarianiL,DomontJ,ColliniP,LePéchoux C,etal.Desmoid-typefibromatosis:afront-lineconservative approachtoselectpatientsforsurgicaltreatment.AnnSurg Oncol.2009;16:2587–93.
8. PikaarA,NortierJW,GriffioenG,VasenHF.Desmoidtumors inpatientswithfamilialadenomatouspolyposis.Ned TijdschrGeneeskd.2002;146:1355–9.
9. LealRF,SilvaPV,AyrizonoMdeL,FagundesJJ,AmstaldenEM, CoyCS.Desmoidtumorinpatientswithfamilial
adenomatouspolyposis.ArqGastroenterol.2010;47:373–8.
10.SchiesslingS,KihmM,GanschowP,KadmonG,BuchlerMW, KadmonM.Desmoidtumourbiologyinpatientswithfamilial adenomatouspolyposiscoli.BrJSurg.2013;100:694–703.
11.FallenT,WilsonM,MorlanB,LindorNM.Desmoidtumors–a characterizationofpatientsseenatMayoClinic1976–1999. FamCancer.2006;5:191–4.
12.BertarioL,RussoA,SalaP,EboliM,GiarolaM,D’AmicoF,etal. Genotypeandphenotypefactorsasdeterminantsofdesmoid tumorsinpatientswithfamilialadenomatouspolyposis.IntJ Cancer.2001;95:102–7.
13.FriedlW,CaspariR,SengtellerM,UhlhaasS,LambertiC, JungckM,etal.CanAPCmutationanalysiscontributeto therapeuticdecisionsinfamilialadenomatouspolyposis? Experiencefrom680FAPfamilies.Gut.2001;48:515–21.
14.SinhaA,TekkisPP,GibbonsDC,PhillipsRK,ClarkSK.Risk factorspredictingdesmoidoccurrenceinpatientswith familialadenomatouspolyposis:ameta-analysis.Colorectal Dis.2011;13:1222–9.
15.LefevreJH,ParcY,KerneisS,GoasguenN,BenisM,ParcR, etal.Riskfactorsfordevelopmentofdesmoidtumoursin familialadenomatouspolyposis.BrJSurg.2008;95:1136–9.
16.ChurchJ,XhajaX,LaGuardiaL,O’MalleyM,BurkeC,Kalady M.Desmoidsandgenotypeinfamilialadenomatous polyposis.DisColonRectum.2015;58:444–8.
17.NieuwenhuisMH,LefevreJH,BulowS,JarvinenH,BertarioL, KerneisS,etal.Familyhistory,surgery,andAPCmutationare riskfactorsfordesmoidtumorsinfamilialadenomatous
polyposis:aninternationalcohortstudy.DisColonRectum. 2011;54:1229–34.
18.SturtNJ,GallagherMC,BassettP,PhilpCR,NealeKF, TomlinsonIP,etal.Evidenceforgeneticpredispositionto desmoidtumoursinfamilialadenomatouspolyposis independentofthegermlineAPCmutation.Gut. 2004;53:1832–6.
19.FearnheadNS,BrittonMP,BodmerWF.TheABCofAPC.Hum MolGenet.2001;10:721–33.
20.CoutureJ,MitriA,LagaceR,SmitsR,BerkT,BouchardHL, etal.Agermlinemutationattheextreme3′endoftheAPC
generesultsinaseveredesmoidphenotypeandisassociated withoverexpressionofbeta-catenininthedesmoidtumor. ClinGenet.2000;57:205–12.
21.GroenEJ,RoosA,MuntingheFL,EntingRH,deVriesJ, KleibeukerJH,etal.Extra-intestinalmanifestationsof familialadenomatouspolyposis.AnnSurgOncol. 2008;15:2439–50.
22.MartinsS,LeiteJ,OliveiraA,SáA,Castro-SousaF.Tratamento dostumoresdesmoidesintra-abdominaisassociadosà PoliposeAdenomatosaFamiliar.RevPortCir.2015;32:17–25.
23.VidaPerezL,MartinezRivasF.Intraabdominaldesmoid tumors.MedClin.2013;141:314–9.
24.Palacios-ZertucheJT,Cardona-HuertaS,Juarez-GarciaML, Valdes-FloresE,Munoz-MaldonadoGE.Casereport:rapidly growingabdominalwallgiantdesmoidtumourduring pregnancy.CirCir.2016,
http://dx.doi.org/10.1016/j.circir.2016.04.004.
25.WongSL.Diagnosisandmanagementofdesmoidtumorsand fibrosarcoma.JSurgOncol.2008;97:554–8.
26.SakorafasGH,NissotakisC,PerosG.Abdominaldesmoid tumors.SurgOncol.2007;16:131–42.
27.BasdanisG,PapadopoulosVN,PanidisS,TzevelekiI, KaramanlisE,MekrasA,etal.Desmoidtumorofmesentery infamilialadenomatouspolyposis:acasereport.Tech Coloproctol.2010;14:S61–2.
28.ChurchJ,LynchC,NearyP,LAGuardiaL,ElayiE.Adesmoid tumorstagingsystemseparatespatientswith
intraabdominal,familialadenomatouspolyposis-associated desmoiddiseasebybehaviorandprognosis.DisColon Rectum.2008;51:897–901.
29.BocaleD,RotelliMT,CavalliniA,AltomareDF.Anti-oestrogen therapyinthetreatmentofdesmoidtumours:asystematic review.ColorectalDis.2011;13:e388–95.
30.OhashiT,ShigematsuN,KameyamaK,KuboA.Tamoxifenfor recurrentdesmoidtumorofthechestwall.IntJClinOncol. 2006;11:150–2.
31.JenayahAA,BettaiebH,SaoudiS,GharsaA,SfarE,BoudayaF, etal.Desmoidtumors:clinicalfeaturesandtreatment options:acasereportandareviewofliterature.PanAfrMed J.2015;21:93.
32.ChaoAS,LaiCH,HsuehS,ChenCS,YangYC,SoongYK. Successfultreatmentofrecurrentpelvicdesmoidtumour withtamoxifen:casereport.HumReprod.2000;15:311–3.
33.NaganoS,PassosR,SantanaM,GuedesV.Tumordesmoide– Umarevisãodeliteratura.RevPatTocantins.2015;2:2–7.
34.NuyttensJJ,RustPF,ThomasCR,TurrisiAT.Surgeryversus radiationtherapyforpatientswithaggressivefibromatosisor desmoidtumors.Cancer.2000;88:1517–23.
35.TurinaM,PavlikCM,HeinimannK,BehrensmeierF,Simmen HP.Recurrentdesmoidsdetermineoutcomeinpatientswith Gardnersyndrome:acohortstudyofthreegenerationsofan APCmutation-positivefamilyacross30years.IntJColorectal Dis.2013;28:865–72.
36.OkunoSH,EdmonsonJH.Combinationchemotherapyfor desmoidtumors.Cancer.2003;97:1134–5.
methotrexateinapatientwithfamilialadenomatous polyposis.ClinJGastroenterol.2009;2:170–4.
38.ChughR,WathenJK,PatelSR,MakiRG,MeyersPA,Schuetze SM,etal.Efficacyofimatinibinaggressivefibromatosis: resultsofaphaseIImulticenterSarcomaAlliancefor ResearchthroughCollaboration(SARC)trial.ClinCancerRes. 2010;16:4884–91.
39.NieuwenhuisMH,Mathus-VliegenEM,BaetenCG,Nagengast FM,vanderBijlJ,vanDalsenAD,etal.Evaluationof managementofdesmoidtumoursassociatedwithfamilial adenomatouspolyposisinDutchpatients.BrJCancer. 2011;104:37–42.
40.JungWB,KimCW,KimJC.Clinicalcharacteristicsadequate treatmentoffamilialadenomatouspolyposiscombinedwith desmoidtumors.CancerResTreat.2014;46:366–73.
41.FisherC,ThwayK.Aggressivefibromatosis.Pathology. 2014;46:135–40.
42.OkunoS.Theenigmaofdesmoidtumors.CurrTreatOptions Oncol.2006;7:438–43.
43.VitellaroM,SalaP,SignoroniS,RadiceP,FortuzziS,CivelliEM, etal.Riskofdesmoidtumoursafteropenandlaparoscopic
colectomyinpatientswithfamilialadenomatouspolyposis. BrJSurg.2014;101:558–65.
44.McNicolFJ,KennedyRH,PhillipsRK,ClarkSK.Laparoscopic totalcolectomyandileorectalanastomosis(IRA),supported byanenhancedrecoveryprogrammeincasesoffamilial adenomatouspolyposis.ColorectalDis.2012;14:458–62.
45.SpeakeD,EvansDG,LallooF,ScottNA,HillJ.Desmoid tumoursinpatientswithfamilialadenomatouspolyposis anddesmoidregionadenomatouspolyposiscolimutations. BrJSurg.2007;94:1009–13.
46.SturtNJ,PhillipsRK,ClarkSK.High-dosetamoxifenand sulindacasfirst-linetreatmentfordesmoidtumors.Cancer. 2004;101:652.
47.MendenhallWM,ZloteckiRA,MorrisCG,HochwaldSN, ScarboroughMT.Aggressivefibromatosis.AmJClinOncol. 2005;28:211–5.
48.DurnoC,MongaN,BapatB,BerkT,CohenZ,GallingerS.Does earlycolectomyincreasedesmoidriskinfamilial