ISSN 0104-6632 Printed in Brazil
www.abeq.org.br/bjche
Vol. 33, No. 02, pp. 373 - 382, April - June, 2016 dx.doi.org/10.1590/0104-6632.20160332s20140077
Brazilian Journal
of Chemical
Engineering
MATHEMATICAL MODELING OF BATCH
ADSORPTION OF MANGANESE ONTO
BONE CHAR
M. E. Maria and M. B. Mansur
*Departamento de Engenharia Metalúrgica e de Materiais, Universidade Federal de Minas Gerais, Av. Antônio Carlos, 6627, Escola de Engenharia, Bloco 2, Sala 3634, Pampulha,
CEP: 31270-900, Belo Horizonte - MG, Brazil. Phone: + (55) (31) 3409-1811, Fax: + (55) (31) 3409-1815
E-mail: marcelo.mansur@demet.ufmg.br
(Submitted: September 25, 2014 ; Revised: January 7, 2015 ; Accepted: March 20, 2015)
Abstract - The present study investigated the dynamics of batch adsorption of manganese onto bone char by using two distinct mathematical formulations: the diffusion model and the shrinking core model. Both models assumed spherical particles and adequately described the transient behavior of metal adsorption under changing operating conditions. Comparatively, the diffusion model described the manganese adsorption better at distinct particle sizes even when small particles were used (dp≤ 0.147 mm); the shrinking core model proved to be more reliable when larger adsorbent particles were used (dp > 0.147 mm), and it described experimental data better at changing solid-liquid ratios. Manganese adsorption was favored when: (i) smaller adsorbing particles were used due to the increase in the contact area and easier access to reacting sites of the char; however, such an effect proved to be limited to dp≤ 0.147 mm, and (ii) higher solid-liquid ratios were used due to the increase in the available reacting sites. External and intraparticle mass transfer dependences on particle size and solid-liquid ratio were also investigated, and results corroborated with prior investigations found in the literature.
Keywords:Adsorption; Manganese; Bone char; Diffusion model; Shrinking core model.
INTRODUCTION
The treatment of industrial effluents containing dissolved metals is commonly done using chemical precipitation, which is a relatively simple operation and an efficient method to attend to legal require-ments. The method, however, is not advantageous in the treatment of sulfuric aqueous solutions contain-ing manganese, as is the case of acid rock drainage (ARD) effluents generated in the southeastern region of Brazil, because manganese presents high solu-bility within a wide range of pH (Bamforth et al., 2006). In fact, the precipitation of manganese may occur at high pH values of approximately 10-11.
After the manganese has been removed, the pH of the effluent must be neutralized in order to discharge the treated water; therefore, the consumption of rea-gents is considerable. In addition, the quantity and the toxicity of the sludge will depend on the type and concentration of the dissolved metals. Therefore, it may result in a costly operation.
The removal and/or recovery of manganese from a typical nickel laterite waste solution was evaluated by Zhang et al. (2010) using distinct precipitation agents, such as hydroxides, carbonate, and SO2/air
precipita-374 M. E. Maria and M. B. Mansur
Brazilian Journal of Chemical Engineering
tion was adequate for the removal/recovery of a large proportion of Mn(II), followed by oxidative precipi-tation applied to reduce manganese concentrations to a very low level. A literature review covering various separation and recovery methods, including solvent extraction, ion exchange, as well as hydroxide, car-bonate, sulfide, and oxidative precipitations, is pre-sented by Zhang and Cheng (2007). These methods are briefly compared and assessed in terms of their selectivity, efficiency, reagent costs, and product quality.
The adsorption of metals onto low cost sorbents, such as bone char, has also been evaluated as an alternative method to treat effluents (Choy and McKay, 2005; Giraldo and Moreno-Piraján, 2008; Kumar et al., 2010; Moreno et al., 2010; Martins et al., 2014; Vieira et al., 2014; Sicupira et al., 2014). Manganese, as well as cadmium, copper, iron, nickel and zinc, could be efficiently removed from sulfuric solutions at nearly neutral pH. As bone char consists basically of hydroxyapatite (Ca10(PO4)6(OH)2) and
calcite (CaCO3), it also raises the pH of the aqueous
solution due to the dissolution of the calcite, thus contributing to a reduction in the lime consumed in the current treatment of ARD solutions containing manganese by means of chemical precipitation. In fact, the efficiency of metal adsorption is not favored in acidic medium due to proton competition at pH < 5.
Experimental results obtained by Moreno et al. (2010) and Sicupira et al. (2014) using ARD efflu-ents revealed that manganese adsorption onto bone char followed satisfactorily the Langmuir equilib-rium isotherm, with a value of qm between 22 and 30 mg g-1. This proved to be a chemisorption process, which is strongly influenced by operating variables, such as the pH of the aqueous phase and the solid/ liquid ratio. Regarding its dynamics, manganese adsorption follows a pseudo-second order kinetic model (Ho, 2006). In addition, data fitting to simpli-fied intraparticle diffusion models, such as those of Weber and Morris (1963), revealed that manganese diffusion within particles is the main rate-limiting step, whereas external mass transfer and intraparticle diffusion phenomena may also affect the removal of manganese when particles of smaller sizes are used.
In an attempt to evaluate the dynamics of manga-nese adsorption onto bone char and to identify the main phenomena that affect the process as a whole, two distinct models were developed in the present paper. In the diffusion model, the transient concen-tration of manganese is assessed by differential mass balances in the external aqueous phase and inside particles (Costodes et al., 2003). According to this model, both intraparticle and chemical reaction rates
may occur simultaneously. Hence, metal concentra-tion inside the particles depends on time and space. The diffusion model consists of a system of partial differential equations to be solved numerically. In the shrinking core model, two distinct regions (named reacted, or ash region, and non-reacted region) may exist inside particles, and the reaction front moves towards the center of the particle, while adsorption proceeds. In this case, metal concentration depends solely on time. The model consists of a system of ordinary differential equations whose numerical so-lution is much simpler to be obtained. However, the diffusion model is classical and it is normally applied to describe adsorption processes. In this context, the aim of the present work is evaluate how adequate the shrinking core model could describe manganese adsorption. A diffusion model was also developed for comparison purposes.
DEVELOPMENT OF BATCH ADSORPTION MODELS
To model the manganese adsorption onto bone char in a batch operation, the following assumptions were formulated: (i) the aqueous phase is an isother-mal and incompressible fluid containing manganese at a known initial concentration and pH; (ii) the po-rous adsorbent particles of bone char are perfectly spherical, containing reacting sites that are homoge-neously distributed within them; (iii) the reaction of manganese in the bone char particles is governed by a chemisorption mechanism (Sicupira et al., 2014); (iv) equilibrium is described by the Langmuir ad-sorption isotherm (Sicupira et al., 2014); (v) the sys-tem is perfectly mixed, so the external mass transfer process occurs solely in a thin boundary layer sur-rounding the particles, and (vi) the pH of the external phase is constant with time due to the buffer effect of calcite dissolution from the bone char.
Diffusion Model (DM)
The mass-balance equation of manganese in the bulk solution is given as follows:
p
e S i r R
dC
k A C C dt
(1)
of concentration, and (ii) the rate of adsorption of manganese is due to the chemical reaction that occurs at reacting sites that are homogeneously distributed within the particle (Crank, 1975).
2 2
ef
i i
a
D
C C q
r
t r r r t
(2)
The equilibrium relation between q and Ci is given by the Langmuir adsorption isotherm (Sicupira
et al., 2014):
1
m i i
aq C q
aC
(3)
The reaction by which the immobilized manga-nese is formed proceeds quite rapidly when com-pared with the diffusion process, and for this reason the kinetics are assumed to be instantaneous and not involved in the rate-controlling process (Teixeira et al., 2001; Lee and McKay, 2004; Jena et al., 2004); therefore, local equilibrium can be assumed to exist between the free Ci(r,t) and immobilized q(r,t) com-ponents of the diffusing manganese species. Hence, deriving Eq. (3) and substituting into Eq. (2):
2
(1 )
i m i
i i
C aq C q q
t C t aC t
(4)
2
2 2
1
ef
a m i i
i
D
aq C C r t r r r aC
(5)
Equations (1) and (5) are subjected to the follow-ing initial and boundary conditions:
0 0C C C ri
,0 0 (6)0
0
i r
C r
(7)
p p
i
ef e i r R
r R
C
D k C C r
(8)
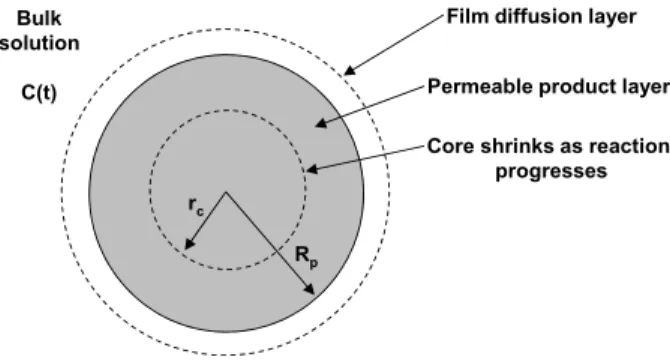
Shrinking Core Model (SCM)
According to this model, the adsorbing particle is surrounded by an external film and consists of two distinct regions schematically shown in Figure 1: the
permeable product layer, which is loaded in manga-nese (Rp≤r < rc), and the unreacted core, which shrinks uniformly as the reaction progresses (rc≤r≤ 0).
Figure 1: Spherical particle representation using the shrinking core model.
The reaction between Mn2+ ions and the reactant solid B of the char is assumed to be irreversible and occurs solely at the product-reactant interface (r = rc) according to the following stoichiometric reaction:
) (
2
(aq) (s immobilized)
Mn b B MnB
(9)
The model assumes that a pseudo steady-state ap-proximation is valid and that the driving force in both external and particle mass transfer is linear, while the driving force in the reaction core incorpo-rates the Langmuir isotherm. In the adsorbing parti-cle, the relative effect of each resistance may change as the reaction proceeds because the length of the permeable product layer increases with time. If all mechanisms occur simultaneously, the system should be handled accordingly and the following rate ex-pressions for each stage (film diffusion, product layer diffusion, and chemical reaction, respectively) can be formulated (Levenspiel, 1999; Han, 2002):
2
2
( ) 4
4
1 1
4
p
p c
c
p e i r R
ef i r R ir r
c p
c r i r r
m
N t R k C C
D C C
r R
q r k C
a q q
(10)
Bulk solution
C(t)
rc
Rp
Film diffusion layer
Permeable product layer
376 M. E. Maria and M. B. Mansur
Brazilian Journal of Chemical Engineering
The adsorption rate N(t) is related to the differen-tial mass balance over the system by equating the decrease in manganese concentration in the solution with the accumulation of the adsorbate in the bone char and the mass balance on a spherical element of adsorbate particle as given by, respectively:
2
4
( ) dC dq rc qm drc
N t V W
dt dt b dt
(11)
Depending on which step of Equation (10) is slow-est, that step is limiting for the overall adsorption process. Three limiting steps may occur: (i) diffusion through the film boundary layer (in this case,
p
i r R
C = 0), (ii) diffusion through the porous product layer (in this case,
p
i r R
C = C and
c
i r r
C = 0), and
(iii) chemical reaction at the reactant-product inter-face (in this case,
c
i r r
C = C). For each case, an
equation giving the time required for a reaction to proceed from particle radius Rp to rc can be obtained by integrating drc/dt with time from 0 to t (Han, 2002). If all mechanisms occur simultaneously, the mass-balance equation for manganese in the bulk solution is:
2
2
2
4
1
p
m
p c p p
e c ef c r
R q C
V a q q dC
dt R r R R k r D r k
(12)
the average loading of manganese in the char is:
2
2
2
4
1
p
m
p c p p
e c ef c r
R q C
W a q q dq
dt R r R R k r D r k
(13)
and the temporal variation of the reacting-core radius is given by:
2
2
1
m m
c
p c c c
p ef r p e
bC q C
q a q q dr
dt r R r r R D k R k
(14)
Equations (12)-(14) were subjected to the follow-ing initial conditions:
0 0C C q0 rc
0 Rp (15)According to this model, the adsorption rate of manganese onto bone char is controlled by the re-spective resistances to film diffusion, product layer diffusion, and chemical reaction. If an instantaneous reaction within spherical adsorbent particles prevails, the third resistance of Eqs. (12)-(14) is null.
Numerical Solution of Models and Estimation of Parameters
Both models were solved numerically using MATLAB software (Version 7.0). The diffusion model, Eqs. (1), (5)-(8), contains a partial differential equation, which was discretized using the implicit finite difference method. Other numerical methods, such as Crank-Nicholson’s implicit finite difference method; a semi-analytical solution method; and the Cartesian collocation method were compared else-where in terms of accuracy, stability, convergence, and computation in CPU time (Lee and McKay, 2004; McKay, 2001). The solution of the shrinking core model, Eqs. (12)-(15), was obtained using the fourth-order Runge-Kutta method. The same numeri-cal solution was used by Sarkar and Bandyopadhyay (2011) and Jena et al. (2004). Properties such as accuracy, stability, convergence and computational time of the numerical methods used in the present work are available elsewhere (Hoffman, 1992; Pinto and Lage, 2001).
Experimental data obtained by Sicupira et al. (2014) using laboratory solutions containing manga-nese were fitted to both models in an attempt to as-sess the values of mass transfer and chemical reac-tion rate parameters under changing operating condi-tions. The estimation of parameters was done nu-merically by minimizing an objective function (F) using an optimization routine found in MATLAB software based on the Nelder-Mead simplex direct search method (Lagarias et al., 1998). The objective function adopted in this work is the sum of the squared deviation between estimated (Cest) and ex-perimental (Cexp) concentrations of manganese in the external aqueous phase:
exp 2
, exp, 1
N
est j j j
F C C
cu ti r w fi re ch co co T ac T th m it th The relativ ulated to me ions are to th
elative error RE Physical c was evaluated ilm diffusion eaction resi hanging the oefficient (k
oefficient (D
The simulatio ccording to Table 1. Typ
he external manganese co
t reached a c he operating
ve error in te easure how he experimen exp exp 1 100 N j N
ESULTS AND consistency o d by simulati n, product la stances. Ca magnitude oke), the intra
Def), and the ons shown in the operatin ical transien aqueous ph oncentration condition of conditions.
erms of perce close estima ntal concentra , exp exp, est j j C C C D DISCUSS
of the shrink ing the indiv ayer diffusion alculations w of the externa aparticle effe reaction rat n Figure 2 w ng condition nt concentrat hase were re diminished f equilibrium
entage was c ated concent ations:
,j
(
SION
king core mo vidual effects n and chemi were done al mass trans ective diffus te constant ( were perform ns portrayed
tion profiles eproduced, i with time un m, depending cal- tra-17) odel s of ical by sfer sion (kr). med d in s in i.e., ntil on Fig mo and sis Ch Def C0 Ta ad et sor ma tio as fro liq par pel etc nal par ob sur tio suc ma val dif eff rev tio tha
gure 2: Sen odel: (a) Film
d kr = 10-5 stance (ke = 1 hemical reac ef = 10-10 m2 s = 100 mg L
able 1: Oper dsorption of
al., 2014).
Param Initial concentr manganese in t Volume of aqu Surface area of Stoichiometric Particle porosit Real density of
Intraparticle rption proces ass transfer r ons simulated shown in Fi om or to sol quid are affec
rticle diamet ller, clearan c. The smalle
l resistance rticles; cons tained. Jadh rvey of the ons and the o
ch mass tra ass transfer lue of Def de ffusion as sh fect of chem veals that fa on. This effec
an those obs
nsitivity anal m diffusion re
m s-1), (b) P 10-5 m s-1 an ction resista s-1) (S/L = 2/4 L-1; pHi = 5.7
rating condi manganese
meter ration of the external pha ueous phase
f bone char constant ty f particle e resistance sses; howeve resistance is d herein, ma igure 2(a). E lid particles cted by a num ter, viscosity
ce, agitation er the value
for mangane sequently, a hav and Pang literature, i observed effe ansfer coeff resistance ecreases, slow hown in Fig mical reactio
ster kinetics ct, however, served for m
ysis of the s esistance (Def Product layer nd kr = 10-5 m ance (ke = 1 400 g mL-1; d
6).
itions of sim onto bone c
Symbol
ase
C0 m
V ap m
b
g
normally go er, the effect
significant ainly when k
External mass suspended mber of vari y of the liqui n speed, ves of ke, the hig ese to reach lower adso garkar (1991 including va ects of typica ficients. The also increas wing down t gure 2(b). An
on shown in favor mang , is much les mass transfer
shrinking co ef = 10-10 m2
r diffusion r m s-1), and ( 0-5 m s-1 an
dp = 0.833 mm
mulated batc char (Sicupir
Unity Value mg L-1 100
L 0.4
m2 g-1 93 - 1 - 0.7975 g cm-3 2.9
overns the a of the extern for the cond
ke < 10-5 m s s transfer rat in an agitate iables, such id, type of im ssel geometr gher the exte the adsorbe orption rate 1) presented arious correl
al variables o e intrapartic ses when th the mangane
nd finally, th n Figure 2( ganese adsor ss pronounce
37 si th th o in (D n ar re k m to in it ad ef h li T sh (S D 0 0 F m 1 it ex an d co 78 imulated con he effect of he diffusion p
The effect f manganese ncluding sim DM) and the
ation coeffic re displayed esulted in a inetics is qui mainly if sma o a larger con ng sites of th t reaches equ
dsorption ki ffect at long avior shown imited when p
Table 2: Fitt hrinking co
S/L = 2/400
Diameter of particle (mm) De c < 0.053 0.104-0.147 0.417-0.833
Figure 3: Eff manganese on
00 mg L-1; p By compa t can be seen
xperimental nd relative e iameters we onditions, a
nditions. As e instantaneou process. t of particle s
e onto bone mulations u e shrinking co cients and rel
in Table 2. D shorter time ite rapid at th aller particle
ntact area an he char, and
uilibrium. T netics at sho er times. Ac n in Figure 3 particles with
ting parame ore models f g mL-1; C0 =
Diffusion mo etermination
coefficient (R2)
R
0.9701 0.9719 0.9731
fect of partic nto bone cha pHi= 5.76).
aring the sim n that the dif transient beh rror < 15%), ere used (dp
sharp decre
evidenced by us reaction is
size on the b char is show using the di
ore model (S lative errors f Decreasing t e to reach eq he beginning s are used, w nd easier con
slows down The particle
orter times, ccording to th 3, however, s
h dp≤ 0.147
eters of the for different = 100 mg L-1
del Shrin Relative error (%) Determ coef (R 14.2 0.9 13.7 0.9 13.9 0.9
cle size on th ar (S/L = 2/40
mulations from ffusion mode
havior quite , even when
dp ≤ 0.147 ease in the c
M. E.
Brazilian Jou
y Crank (197 s to slow do
batch adsorpt wn in Figure
iffusion mo SCM). Determ
for both mod the particle s quilibrium. T g of the proce which is rela ntact with rea
over time u size affects but it has li he transient such effects mm were use
diffusion a t particle si
1
; pHi = 5.76
nking core mod mination fficient
R2)
Relat erro (% 9667 18. 9626 19. 9972 2.9 he adsorption 00 g mL-1; C
m both mod el described
well (R2 > 0 smaller parti mm). In th oncentration
Maria and M. B.
urnal of Chemica
75), own tion e 3, odel mi-dels size The ess, ated act-ntil the ittle be-are ed. and izes 6). del tive or %) 3 7 9 n of
C0 =
els, the 0.97 icle hese n of ma aft suc err po wh the we shr wh
≥ 0
res per the inc a l the kin of cor 0.9 mo bec the Ta sh rat 5.7 Fig tio mm So ( . Mansur al Engineering anganese oc terwards. Th ch behavior ror < 20%).
orer than th hen small pa e unreacted a ell defined i
rinking core hen larger pa 0.417 mm, w Regarding sults portray
rcentage of m e increase i crease in the onger time w e increase in netics of ma first contac re model des 99 and relat odel (R2 > 0. cause larger ese runs (Tab
able 3: Fitti rinking cor tios (dp = 0.4 76).
gure 4: Effe on of mangan m; C0 = 100
olid-liquid ratio (g mL-1)
Dete co
1/400 2/400 3/400
ccurred at s he shrinking satisfactoril However, hat obtained articles were and reacted in smaller d e model pro articles of th with R2 > 0.99
the effect yed in Figur
manganese r in the solid
quantity of r was required the solid-liq anganese ads ct. In these s
scribed data tive error <
95 and relati particles of t ble 3).
ng paramet re models f 417-0.833 mm
ect of solid-l nese onto bo mg L-1; pHi
Diffusion mod ermination oefficient
(R2) Re
er ( 0.9833 0.9731 1 0.9597 3
shorter time core model ly (R2 > 0.9 fitting was d by the dif used, most regions migh dimensions. T oved to be he adsorbent 9 and relativ of solid-liqu re 4 reveal removal was d-liquid ratio
reacting char to load the a quid ratio, des orption iden simulations, comparative 10%) than ive error < 3 the adsorben
ters of the d for differen
m; C0 = 100
liquid ratio o one char (dp
= 5.76). del Shrink elative rror (%) Determi coeffic (R2
2.8 0.99 13.9 0.99 32.8 0.99
es, stabilizin also describe 6 and relativ comparative ffusion mod likely becau ht not be ve Therefore, th
more reliab were used ( e error < 3% uid ratio, th
that a high obtained wi o due to th r. As expecte adsorbent wi spite the fast ntified at tim the shrinkin ely better (R2
the diffusio 5%), probab nt were used
diffusion an nt solid-liqu
mg L-1; pHi
on the adsor = 0.417-0.83 king core mode
ination cient 2) Relativ error (%) 907 1.8 972 2.9 967 8.4 ng ed ve ely del use ry he ble (dp %). he her ith he ed, ith ter mes ng 2 > on bly in nd id
i =
d ou g in in p at th ra la th so ar (n st (D d b ef th in m re o ca 5 in A Ja m sp 2 w D m 5 is ti m u ca o b o F w co o (1 w m The transp ata fitting u us reaction t anese onto nvestigated b n Figure 5. T arameters w ting conditio he same resu ameters. Pow ate data of th he investigat ome cases th re commonly no preferred tructures suc Do and Ngu
iffusion mod etter, while ffect of the he estimates ncluded in F model with a epresented b ther is repres The averag ally obtained (a). Such a r nvestigations Asai et al.,
adhav and P mass transfer
phere to an in (Sherwood with molecul
DMn,water = 6.8 menico and S (a). The pos s attributed ively higher models were
sed (dp ≤ 0. ant decrease f manganese ents. Concer n the extern Figure 5(b), i with an avera onsidering e rating the e 1988).
An opposi was identifie model (Figur
port coefficie sing both m to describe th
bone char i by Sicupira The effect of
as not prono on, the soluti ult with diff wer law equa he transport c
ted operatin he R2-values y used to de d length scal ch as fracta uyen, 1988).
del described the shrinking solid-liquid obtained by Figure 5 for
higher deter by a wide co
sented by a n ge dependen d for both m result corrobo s (Harriot, 1 1988; Arme Pangarkar, 19 coefficients nfinite stagn number is g lar diffusivit 8x10-10 m2 s -Schwartz, 199
sitive deviati to the conv deviations b
observed w .147 mm), th in the exter e when micro
rning the eff nal mass tra it was found age value of k
stimates from experimenta
ite behavior w ed by data
re 5(c)). Ex
ents ke and D models assum
he batch adso in the opera
et al. (2014 f correlation unced, since ion practicall ferent initial ations were c coefficients a ng condition
were poor, escribe self-s le), as verifi als and adso As previou d the effect g core mode d ratio better y using both r comparison rmination co ontinuous cu narrow dashe nce of ke on d models, as sh orates a num 1962; Kubo enante and 991). Values of mangane nant fluid, wh given by Sh =
ty of manga
-1
at 25 oC, ac 98) is also sh on from this vective cont etween estim when small hus evidenc rnal mass tra oparticles are fect of the so ansfer coeffic d to be of lit
ke = (13±4)x m both mode l findings o
with depend fitting using xternal and
Def assessed ming instanta orption of m ating conditio 4) are display n between fit e, for each op ly converged guesses of chosen to cor
as a function ns. Although such equatio similar syste fied in ramif orbent partic usly shown, of particle s el described r. Neverthele
h models w n purposes; oefficient (R2
urve, while ed curve.
dp-0.5 was pra hown in Fig mber of previo
i et al., 19 Kirwan, 19 of the exter ese to or from hich yields S
= kedp/DMn,w anese in wa ccording to D hown in Fig s limiting va tribution. Re mates from b
particles w ing the sign ansfer resistan
e used as ads olid-liquid ra cient shown ttle importan x10-6 m s-1 wh els, thus corr
of Asai et
dence Def d g the diffus
internal m by ane- man-ons yed tted per-d to pa- rre-n of h in ons ems fied cles the size the ess, were the 2 ) is the cti-gure ous 974; 989; rnal m a
Sh = water, ater Do-gure alue ela-both were nifi-nce sor-atio n in nce, hen ob-al.
dp0.6 sion mass tra to par lon ben tai fer liq sho int rat du ansfer resista the boundar rticle resist nger diffusio nt particles ned may be rent class siz quid ratio on own in Figu ternal resistan tios were use ue to the incre
ances are clo ry condition, ance was e on path is exp are used. T attributed to zes. As regar n the intrapar ure 5(d), a s nce was foun ed (dependen
ease in the am
osely connec Eq. (8), so expected. In pected when Therefore, th o diffusion in rds the effec rticle diffusi significant in nd when high nce Def(S/L mount of poro
cted accordin a higher intr n addition, n bigger adso
he results o n pores of di ct of the soli
ion coefficie ncrease in th her solid-liqu
38 F d co op (2 an fe tr tr cr p at T n ch p m w lo en co is li d p d M ad th th to 80
Figure 5: Tran ata fitting w ore model as perating con 2014).
The Biot nalogous to t er) is defined ransfer resista ransfer resist
ropera et al. ore diffusion ting conditio Therefore, th ot uniform th
The batch har was m roaches assu model and th were compare owing conclu
Both m
nt behavior o oncentration shed monoto ibrium, whic
itions;
The di
article size b escribed the Manganese ad
dsorbing par he contact ar he char, but o dp ≤ 0.147
nsport coeffi with the diffu
ssuming insta nditions inve
number for the Biot num d as the ratio ances (Bim = tance within
, 2007). Acc n was found ons investigat he manganes hroughout th CONCL h adsorption modeled usin uming spher he shrinking ed with exp usions were d models adequ of metal ads n in the exte onically over h depended
ffusion mod better, while e effect of th dsorption wa rticles were rea and easie such an effe 7 mm, and (i
icients ke and fusion model antaneous rea estigated by
r mass tran mber for trans
of external t
kedp/Def). If particles is cording to su to predomin ted by Sicupi se concentra he adsorbent
LUSIONS
of mangan ng two ma rical particle g core mode
erimental da drawn: uately descri
orption. In f ernal aqueou r time until i on the given
del described e the shrinki
he solid-liqu as favored w
used due to er access to r ect was foun ii) higher sol
M. E.
Brazilian Jou
d Def assessed l and shrink action under
Sicupira et
nsfer (which sient heat tra to internal m
Bim > 0.1, m significant ( uch a parame nate for all op
ira et al. (201 ation profile
particles.
nese onto bo athematical
s: the diffus el. Simulatio ata and the f
ibed the tran fact, mangan us phase dim
it reached eq n operating c
d the effect ing core mo uid ratio bet when: (i) sma the increase reacting sites nd to be limi lid-liquid rat
Maria and M. B.
urnal of Chemica
d by king the al. h is ans-mass mass (In-eter, per-14). e is one ap-sion ons fol- nsi-nese min- qui- on-of odel tter. ller e in s of ited tios we ing cie sol sen lite do cha and 04 and nan a ap AS b B Bim C C0 Ci Def dp F ke kr N( q qm r Rp R2 rc . Mansur al Engineering
ere used due g sites;
External ents were eva
lid-liquid rat nt work cor erature. Intra
minate the ar under the
A
The authors d the Brazil 026-10), CN d INCT Acq ncial suppor
affinit (L mg surfac superf (m2 m stoich (-) reacta
m Biot n
conce extern initial extern conce adsorb
ef effect
diame object mass t extern reactio system
t) adsorp
conce within theore of Lan radial partic radius determ unreac
to the incre
l and intrapa aluated for c tios. Depend rroborated p aparticle ma
adsorption operating co
CKNOWLE
s wish to ack lian agencie NPq (304788/ qua (www.ac t. NOMENC ty parameter g-1)
ce area of bon ficial area of m-3)
hiometric con
ant solid defi number of m entration of m
nal phase (mg l concentratio nal bulk phas entration of m
bent particle ive diffusion eter of adsorb tive function transfer coef nal phase (m on rate const ms (m s-1)
ption rate at entration of im
n the adsorbe etical maxim ngmuir isoth distance fro le, 0 < r < R
s of adsorben mination coe
cted core rad
ease in the av
article mass t changing par dences assess prior studies ass transfer of mangane onditions inv EDGMENT knowledge PP es FAPEMIG /2012-0), CA cqua-inct.org CLATURE
r of Langmui
ne char parti f adsorbent p
nstant define
ned by Eq. ( ass transfer ( manganese in
g L-1) on of mangan se (mg L-1) manganese w
(mg L-1) n coefficient
bent particle n defined by E
fficient in the s-1)
tant for heter
time t (mg s -mmobilized ent particle (m mum adsorptio
herm (mg g-1) om the center
Rp (m) nt particle (m efficient (-)
dius (m)
vailable reac
transfer coeff rticle sizes an sed in the pr
found in th was found ese onto bon vestigated.
TS
PGEM/UFM G (TEC-APQ APES-PROEX g) for their f
ir isotherm
icles (m2 g-1) particles
d by Eq. (9)
9) (-) (-) n the bulk
nese in the
within the
(m2 s-1) (m) Eq. (16) (-) e bulk
rogeneous
-1
)
manganese mg g-1) on capacity )
r of the
Sh Sherwood number (-)
S/L solid-liquid ratio (g mL-1)
t time (s)
V volume of aqueous phase (L)
W weight of the adsorbent (g)
Greek Symbols
particle porosity (-)
density of adsorbent particle (g m-3) a apparent density of adsorbent particle
(g m-3)
REFERENCES
Armenante, P. M., Kirwan, D. J., Mass transfer to microparticles in agitated systems. Chemical En-gineering Science, 44, 2781-2796 (1989). Asai, S., Konishi, Y., Sasaki, Y., Mass transfer
be-tween fine particles and liquids in agitated ves-sels. Journal of Chemical Engineering of Japan, 21, 107-112 (1988).
Bamforth, S. M., Manning, D. A. C., Singleton, I., Younger, P. L., Johnson, K. L., Manganese re-moval from mine waters - investigating the occur-rence and importance of manganese carbonates. Applied Geochemistry, 21, 1274-1287 (2006). Choy, K. K. H., McKay, G., Sorption of metal ions
from aqueous solution using bone char. Environ-ment International, 31, 845-854 (2005).
Costodes, T., Fauduet, V. C., Porte, H., Delacroix, C. A., Removal of Cd(II) and Pb(II) ions, from aque-ous solutions, by adsorption onto sawdust of Pinus sylvestris. Journal of Hazardous Materials, 105, 121-142 (2003).
Crank, J., The Mathematics of Diffusion. 2nd Ed., Clarendon Press, Oxford (1975).
Do, D. D., Nguyen, T. S., A power law adsorption model and its significance. Chemical Engineering Communications, 72, 171-185 (1988).
Domenico, P. A., Schwartz, F. W., Physical and Chemical Hydrogeology. 2nd ed., John Wiley & Sons, USA (1998).
Giraldo, L., Moreno-Piraján, J. C., Pb2+ adsorption from aqueous solutions on activated carbons ob-tained from lignocellulosic residues. Brazilian Journal of Chemical Engineering, 25(1), 143-151 (2008).
Han, K. N., Fundamentals of aqueous metallurgy. SME, USA (2002).
Harriott, P., Mass transfer to particles – Part I: Sus-pended in agitated vessels. AIChE Journal, 8, 93-102 (1962).
Ho, Y. S., Review of second-order models for ad-sorption systems. Journal of Hazardous Materials, B136, 681-689 (2006).
Hoffman, J. D., Numerical Methods for Engineers and Scientists. McGraw-Hill, New York, USA (1992).
Incropera, F. P., Dewitt, D. P., Bergman, T. L., Lavine, A. S., Fundamentals of Heat and Mass Transfer. 6th Ed., John Wiley & Sons, USA (2007).
Jadhav, S. V., Pangarkar, V. G., Particle-liquid mass transfer in mechanically agitated contactors. In-dustrial & Engineering Chemistry Research, 30, 2496-2503 (1991).
Jena, P. R., Basu, J. K., De, S., A generalized shrink-ing core model for multicomponent batch adsorp-tion process. Chemical Engineering Journal, 102, 267-275 (2004).
Kuboi, R., Komasawa, I., Otake, T., Iwasa, M., Fluid and particle motion in turbulent dispersion – III: Particle liquid hydrodynamics and mass transfer in turbulent dispersion. Chemical Engineering Science, 29, 659-668 (1974).
Kumar, P. S., Ramakrishnan, K., Kirupha, S. D., Sivanesan, S., Thermodynamic and kinetic stud-ies of cadmium adsorption from aqueous solution onto rice husk. Brazilian Journal of Chemical En-gineering, 27(2), 347-355 (2010).
Lagarias, J. C., Reeds, J. A., Wright, M. H., Wright, P. E., Convergence properties of the Nelder-Mead simplex method in low dimensions. SIAM Jour-nal Optimization, 9, 112-147 (1998).
Lee, V. K. C., McKay, G., Comparison of solutions for the homogeneous surface diffusion model ap-plied to adsorption systems. Chemical Engineer-ing Journal, 98, 255-264 (2004).
Levenspiel, O., Chemical Reaction Engineering. 3rd Ed., John Wiley & Sons, USA (1999).
Martins, R. J. E., Vilar, V. J. P., Boaventura, R. A. R., Kinetic modeling of cadmium and lead removal by aquatic mosses. Brazilian Journal of Chemical Engineering, 31(1), 229-242 (2014).
McKay, G., Solution to the homogeneous surface diffusion model for batch adsorption systems us-ing orthogonal collocation. Chemical Engineerus-ing Journal, 81, 213-221 (2001).
Moreno, J. C., Gómez, R., Giraldo, L., Removal of Mn, Fe, Ni and Cu ions from wasterwater using cow bone charcoal. Materials, 3, 452-466 (2010). Pinto, J. C., Lage, P. L. C., Métodos numéricos em
problemas de engenharia química. E-papers, Rio de Janeiro, Brazil (2001). (In Portuguese).
382 M. E. Maria and M. B. Mansur
Brazilian Journal of Chemical Engineering
69-77 (2011).
Sicupira, D. C., Silva, T. T., Leão, V. A., Mansur, M. B., Batch removal of manganese from acid mine drainage using bone char. Brazilian Journal of Chemical Engineering, 31, 195-204 (2014). Teixeira, V. G., Coutinho, F. M. B., Gomes, A. S.,
The most important methods for the characteriza-tion of porosity of styrene-divinylbenzene based resins. Química Nova, 24, 808-818 (2001). (In Portuguese).
Vieira, M. G. A., de Almeida Neto, A. F., da Silva, M. G. C., Carneiro, C. N., Melo Filho, A. A., Ad-sorption of lead and copper ions from aqueous ef-fluents on rice husk ash in a dynamic system.
Brazilian Journal of Chemical Engineering, 31(2), 519-529 (2014).
Weber, W. J., Morris, J. C., Kinetic of adsorption carbon solution. Journal of Sanitary Engineering Divisor, American Society Civil Engineers, 89, 31-59 (1963).
Zhang, W., Cheng, C. Y., Manganese metallurgy review. Part II: Manganese separation and re-covery from solution. Hydrometallurgy, 89, 160-177 (2007).