w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
Validation
and
psychometric
properties
of
the
EULAR
Sjögren’s
Syndrome
Patient
Reported
Index
(ESSPRI)
into
Brazilian
Portuguese
夽
Maurício
Aquino
Paganotti
a,
Valéria
Valim
b,c,d,∗,
Érica
Vieira
Serrano
e,f,
Samira
Tatiyama
Miyamoto
g,
Raquel
Altoé
Giovelli
e,
Maria
Carmen
Lopes
Ferreira
Silva
Santos
h,iaUniversidadedeVilaVelha(UVV),VilaVelha,ES,Brazil
bRheumatologyProgram,DepartmentofInternalMedicine,UniversidadeFederaldoEspíritoSanto(Ufes),Vitória,ES,Brazil cComissãoBrasileiradeSíndromedeSjögren,Brazil
dSociedadeBrasileiradeReumatologia,Brazil
eDepartmentofInternalMedicine,EscolaSuperiordeCiênciasdaSantaCasadeMisericórdiadeVitória(Emescam),Vitória,ES,Brazil fHospitalUniversitárioCassianoAntonioMoraes(Hucam),UniversidadeFederaldoEspíritoSanto(Ufes),Vitória,ES,Brazil
gPhysicalTherapyCourse,UniversidadeFederaldoEspíritoSanto(Ufes),Vitória,ES,Brazil hDepartmentofPathology,UniversidadeFederaldoEspíritoSanto(Ufes),Vitória,ES,Brazil
iServic¸odeAnatomiaPatológica,HospitalUniversitárioCassianoAntonioMoraes(Hucam),UniversidadeFederaldoEspíritoSanto
(Ufes),Vitória,ES,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received14June2013 Accepted28June2015
Availableonline4September2015
Keywords:
Sjögrensyndrome Scales
Validationstudies ESSPRI
a
b
s
t
r
a
c
t
Objective:Tocarryoutthecross-culturaladaptationofEULARSjögren’sSyndromePatient ReportedIndex(ESSPRI)forPortugueselanguageandevaluateitspsychometricproperties.
Method:Cross-sectionalstudyofpatientswithprimarySjögren’ssyndrome(SS).The psy-chometricproperties(intraobserverreproducibilityandconstructvalidity)werestudied.In constructvalidity,ESSPRIwascomparedwiththePatient’sGlobalAssessment(PGA),Profile ofFatigueandDiscomfort(Profad),SiccaSymptomsInventory(SSI)andFunctional Assess-ment ofChronicIllnessTherapy(Facit-F).Statisticaltestsused were:Cronbach’salpha, intraclass correlationcoefficient (ICC),Bland–AltmanmethodandSpearmancoefficient. Avalueofp≤0.05wasconsideredsignificant.
Results:Therewasnodifferencebetweenversionsinbothlanguages;thus,aBrazilian con-sensualversionwasobtained.Allsubjectswerewomenaged49.4±11.6years,withonset ofsymptomsof7.2±5.4years,andtimeofdiagnosisof3.0±3.3years.ThemeanESSPRI was6.87±1.97.Theintraobserverreproducibilitywashighandsignificant(0.911)and,with Bland–Altmanmethod,therewasnosystematicbiasintheagreementofmeasuresamong evaluations.AmoderatecorrelationofESSPRIwithalltestedinstrumentswasobserved.
夽
StudyconductedatDepartmentofInternalMedicineandServiceofRheumatology,HospitalUniversitárioCassianoAntonioMoraes (HUCAM),UniversidadeFederaldoEspíritoSanto(UFES),Vitória,ES,Brazil.
∗ Correspondingauthor.
E-mail:val.valim@gmail.com(V.Valim). http://dx.doi.org/10.1016/j.rbre.2015.08.001
Conclusion: TheBrazilianPortugueseversionofESSPRIisavalidandreproducibleversion. ©2015ElsevierEditoraLtda.Allrightsreserved.
Validac¸ão
e
propriedades
psicométricas
do
Eular
Sjögren’s
Syndrome
Patient
Reported
Index
(ESSPRI)
para
a
língua
portuguesa
Palavras-chave:
SíndromedeSjögren Escalas
Estudosdevalidac¸ão Esspri
r
e
s
u
m
o
Objetivo: Fazeraadaptac¸ãotransculturaldoEularSjögren’sSyndromePatientReported Index(Esspri)paraalínguaportuguesaeavaliarassuaspropriedadespsicométricas.
Método: EstudotransversaldepacientescomsíndromedeSjögrenprimária(SS).Foram estudadasaspropriedadespsicométricas(reprodutibilidadeintraobservadoreavalidade deconstruto).Navalidadedeconstruto,oESSPRIfoicomparadocomoPatient’sGlobal Assessment(PaGA),ProfileofFatigueandDiscomfort(Profad),SiccaSymptomsInventory (SSI)eFunctionalAssessmentofChronicIllnessTherapy(Facit-fatigue).Ostestes estatís-ticosusadosforamo␣-Cronbach,coeficientedecorrelac¸ãointraclasse(CCI),métodode
Bland-AltmanecoeficientedeSpearman.Foiconsideradosignificativoop≤0,05.
Resultados: Nãohouvediferenc¸aentreasversõesnasduaslínguaseobteve-se,assim,a versãoconsensualbrasileira.Todososindivíduosforammulheresde49,4±11,6anos,com iníciodossintomasde7,2±5,4anosetempodediagnósticode3±3,3anos.Amédiado Essprifoide6,87±1,97.Areprodutibilidadeintraobservadorfoialtaesignificativa(0,911)e, nométododeBland-Altman,nãohouveviéssistemáticonaconcordânciadasmedidasentre asavaliac¸ões.Houvecorrelac¸ãomoderadadoEsspricomtodososinstrumentostestados.
Conclusão: AversãodoEsspriemportuguêséválidaereprodutível.
©2015ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Sjögren’ssyndrome(SS)isanautoimmune,chronicsyndrome with slow and progressive evolution. SS is considered as thesecond mostcommon autoimmune rheumaticdisease, affecting0.17%ofBrazilianpopulation,1similartoother
Euro-peanstudies using the American-Europeancriterion (2002) fordiagnosticclassification.2,3 SSismorphologically
charac-terizedby alymphocytic infiltratein salivaryand lacrimal glands,4,5but20–40%ofpatientspresentextraglandular
man-ifestationsin musculoskeletal, pulmonary, gastrointestinal, hepatic, hematologic, vascular, dermatological, renal, and neurologicalsystems.4–7
Qualityoflifeisimpairedindifferentaspects:physical, psy-chologicalandsocial,asaresultofdrynessandextraglandular manifestations.7–15 AlthoughSSisaprevalentdisease with
greatimpact,thereislittleevidenceforitstreatment.Inthe lastdecade,manynewdrugs,especiallythosepertainingto theclassofbiologicalagents,havebeendevelopedanditis expectedthatthesedrugscanbetestedinthisdisease.Inthis context,theEuropeanLeagueAgainstRheumatism(EULAR) developedthe EULAR Sjögren’sSyndrome Patient Reported Index(ESSPRI),ameasuringinstrumentthathasbeen used asanendpointinclinicaltrialstoevaluatethesubjective per-ceptionofthepatientwithrespecttothemostimportantand frequentsymptoms,suchasfatigue,painanddryness,and theirimpactonthe disease.16,17 Previousinstruments such
astheProfileofFatigueandDiscomfort(PROFAD),18theSicca
SymptomsInventory(SSI)19andtheshortversionofthe
Pro-fileofFatigue and Discomfort– SiccaSymptoms Inventory
(PROFAD-SSI)20havethelimitationofassessingonlyanaspect
ofthisdisease(onlyfatigue/pain,oronlydryness),whereas ESSPRIgatheredinasinglecompositeindexthesethree symp-toms.
ESSPRIiscompletedbythepatientanditcontainsjustthree itemstobegivenanactivitylevelscorebetween0–10:pain, fatigueanddryness,thefinalESSPRIscoreisthemeanofall threescoresandthereforealsobetween0–10.ESSPRIwas pre-liminaryvalidatedbyamulticenterstudyin201116and2014.21
Thisinstrumenthasreproducibility=0.94,anditssensitivity tochangeislow(−0.37),butsignificantlyhigherwhen com-paredtoSSI(p=0.006)andPROFAD(p=0.049).21After16,24,
36and60weeksoftreatmentwithrituximab,ESSPRIshowed significantimprovement(decreaseofthepatient’ssymptoms) comparedtotheinitialevaluation.22
Currently,ESSPRIisbeingextensivelyusedandhasshown a correlation with quality of life23 and functional status24
measures,andcanbeconsideredausefulpredictorofhealth statusofpatientswithpSS.25
Theaimofthisstudywastocarryoutacross-cultural adap-tationofESSPRIintoPortugueselanguageandtoevaluateits psychometricproperties(Annex1).
Patients
and
methods
Patients
The study sample consisted of subjects with SS coming fromtheSjögren’sSyndromeOutpatientClinic,Rheumatology Department,HospitalUniversitárioCassianoAntônioMoraes (HUCAM),inVitória-ES.
Methods
Inclusionandexclusioncriteria
Patients included fulfilled the following criteria: diagnosed withSSaccordingtoEuropean-AmericanCriteria(2002)forthe classificationofthisdisease;agedover18yearsold;andagreed toparticipate.Subjectswithotherautoimmunediseases, cir-rhosis, sarcoidosis, known hepatitis C infection, acquired immunodeficiency syndrome, preexisting lymphoma, graft
versushostdisease;headandneckradiationinthepast;and previoususeofanticholinergicdrugs,wereexcluded.
DomainsandcalculationofESSPRI
TheESSPRIquestionnaireconsistsofthreeitemstobegiven anactivitylevelscorebetween0–10:pain(jointand/or mus-cle pain), fatigue and dryness (0=no symptom at all and 10=worstsymptomimaginable.Thepatientmustcheckthe alternativethatbestdescribestheseverityofhis/her symp-tomsintheworststagesinthelasttwoweeks.Eachdomain representstheseverityofthesymptomindependently; how-ever,itisalsopossibletoobtainafinalscorebyaveragingthe scoresofthethreedomains.
Although the instrument is self-administered, it was decidedinthisstudytouseonlyaface-to-faceinterview,in viewoftheloweducationlevelofparticipants.
Transculturaladaptation
Totesttheequivalence(conceptual,oftheitem,semanticand operational)ofESSPRI,aninternationalmethodologywas fol-lowedtogeneratetheconsensusversionintoPortuguese,26a
methodalsousedbyotherBrazilianauthors.27–31
Psychometricproperties. Thepsychometricpropertiesverified were:internalconsistency,intraobserverreproducibility,and constructvalidity.Totestreproducibility,ESSPRIwasapplied twicebythesameobserver (intraobserverevaluation),with anintervalof2daysbetweenthefirstandthesecond eval-uation,takingintoaccountthatthisisaninstrumentbased onlyonthesubjectiveperceptionofthepatient’ssymptoms. Toaccessconstructvalidity,westudiedthecorrelationpower betweenESSPRIandPROFAD,18SSI,19FunctionalAssessment
ofChronicIllnessTherapy(FACIT-F)andsubjectiveperception ofdiseaseactivitybythepatientthroughtheuseofavisual scale(Patient’sGlobalAssessment–PGA).16
Statisticalanalysis
Tostudythesemanticequivalence,asampleof20patientswas used.Thereisnomathematicalformulaforasample calcula-tionatthisstage,andsmallsamplesareconsideredsufficient forthisqualitativeanalysis.31Asforthestudyof
psychomet-ricmeasurementproperties(validationandreproducibility), thesamplewascalculatedbasedontheuseofatleastfive
patientsperdomainoftheinstrument.32Theminimum
cal-culatedsamplewas15patients.
To evaluate psychometric properties,27–30 the following
analyzes were performed: (1) Cronbach’salphaforinternal consistency;(2)intraclasscorrelationcoefficient(ICC)andthe graphicmethodofBland–Altmanstatisticsforintraobserver reproducibility;(3)SpearmancorrelationcoefficientofESSPRI
versusPGA,PROFAD,SSIandFACIT-Fforconstructvalidity. Instatisticalanalysis,thesoftwareSPSS19.0wasused,and apvalue≤0.05wasconsideredsignificant.
Results
In theevaluationstage ofsemantic equivalence, apre-test withtheconsensualversionofESSPRI(Table1)wascarried out.Thetestwasappliedto20patientsandtherewereno“not
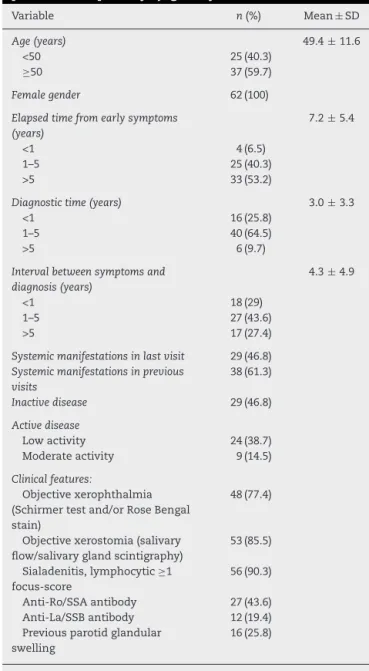
Table1–Clinicalanddemographiccharacteristicsof62 patientswithprimarySjögrensyndrome.
Variable n(%) Mean±SD
Age(years) 49.4±11.6
<50 25(40.3)
≥50 37(59.7)
Femalegender 62(100)
Elapsedtimefromearlysymptoms (years)
7.2±5.4
<1 4(6.5)
1–5 25(40.3)
>5 33(53.2)
Diagnostictime(years) 3.0±3.3
<1 16(25.8)
1–5 40(64.5)
>5 6(9.7)
Intervalbetweensymptomsand diagnosis(years)
4.3±4.9
<1 18(29)
1–5 27(43.6)
>5 17(27.4)
Systemicmanifestationsinlastvisit 29(46.8)
Systemicmanifestationsinprevious visits
38(61.3)
Inactivedisease 29(46.8)
Activedisease
Lowactivity 24(38.7) Moderateactivity 9(14.5)
Clinicalfeatures:
Objectivexerophthalmia (Schirmertestand/orRoseBengal stain)
48(77.4)
Objectivexerostomia(salivary flow/salivaryglandscintigraphy)
53(85.5)
Sialadenitis,lymphocytic≥1 focus-score
56(90.3)
Anti-Ro/SSAantibody 27(43.6) Anti-La/SSBantibody 12(19.4) Previousparotidglandular
swelling
16(25.8)
Table2–Resultsofagreementbetweenevaluationson eachquestionandESSPRItotalscore.
Questions ICC
Dryness 0.773
Fatigue 0.870
Pain 0.770
ESSPRItotalscore 0.911
ICC,intraclasscorrelationcoefficient;ESSPRI,EULARSjögren’s Syn-dromePatientReportedIndex.
understood”questionstomorethan15%ofpatients;thus,this wasconsideredthefinalversionofESSPRIforthePortuguese language.
Sixty-twowomenwithSS wereincludedtoevaluatethe psychometricproperties.Themeanagewas49.4±11.6years, withpredominanceofparticipantsover50yearsold.Asfor ethnicity,43.56%wereBrown,32.25%Caucasian,and24.19% ofAfrican descendent. Themajority (56.41%) had low lev-els ofeducation (<8 years), and 8.06% were illiterate. The durationofdiseasewas7.2±5.4years,46.8%ofpatientshad some systemic manifestation and 87.1% had already used immunosuppressivedrugsinthepast.Mostpatientshadan inactivedisease(46.8%)and38.7%showedlowdisease activ-ity,accordingtothephysician’ssubjectivejudgment(Table1). Thediagnosticclassificationcriteriafrequency6isdescribed
inTable1.
The questionnaire was answered easily by 59.67% of patients(score≤3).Themeandegreeofdifficultytoanswer the ESSPRI questionnaire was 2.84±3.05, on a scale of 0–10. The mean for ESSPRI was 6.87±1.97 on a scale of 0–10.
TheinternalconsistencyofESSPRIwasweak,resultingin aCronbach’salphavaluecorrespondingto0.447.The intra-observer reproducibility for this instrument was high and significant(0.911).WiththeuseoftheBland–Altmanmethod (Fig.1),itwasobservedthattherewasnosystematicbiasin theagreementofthemeasuresbetweeninterviews,because ofthegooddistributionofdatathroughoutitsextension.Most measurementswere distributed withinacceptable limitsof variation,withonlytwopointsoutsidethestandarddeviation
12 10 8 6 4 2 0 –4 –3 –2 –1 0 1 2 3
ESSPRI
E
v
a
lu
a
ti
o
n
1
Evaluation
2
Mean
–0.1
–1.96 SD
–2.6 +1.96 SD
2.4
Fig.1–ConcordanceofmeasuresoffinalESSPRIscore betweenevaluations1and2.Bland–Altmangraph.
rangeof±1.96(outliers),butnearthelimits,indicatingthat measuresbetweeninterviewstendtoproducesimilarresults. ThereproducibilityofeachquestionofESSPRIwastested separately,withhighconcordanceforallquestions(Table2).
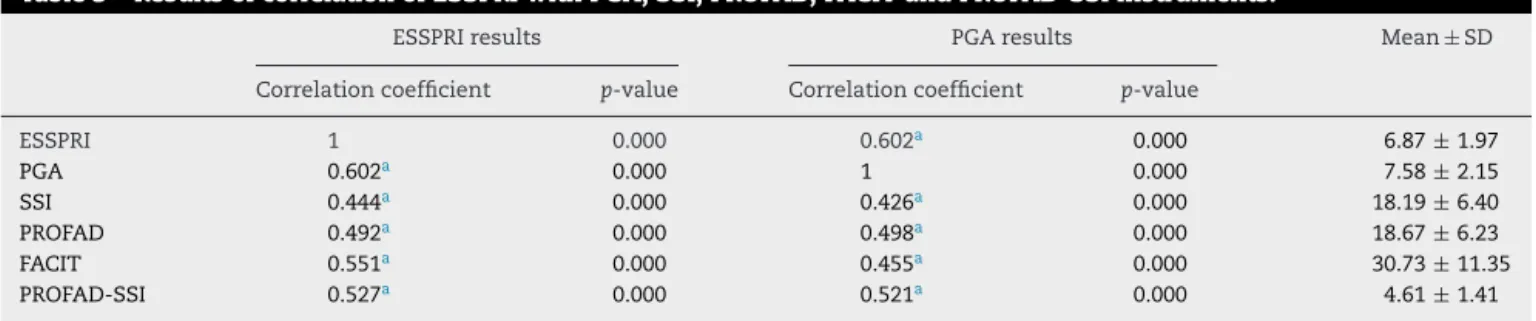
ItisobservedfromTable3theexistenceofamoderateand significantcorrelationofESSPRIwithalltestedinstruments. Thecorrelationwaspositive,thatis,whentheESSPRIscore increases,theotherscoresalsoincrease.
Discussion
Recently,ESSDAI(EULARSyndromeSjögren’sDiseaseActivity Index),aninstrumentforevaluationofdiseaseactivitybased onobjective criteriaandevaluationbythephysician,32 and
PROFAD-SSI,33aninstrumentthatforegoesthedevelopment
ofESSPRI,weretranslatedandvalidatedintoPortuguese lan-guage.However, nowadaysESSPRIisthe mostwidelyused instrument worldwide in the subjective assessment of SS severityofsymptoms,accordingtothepatient’sperception, withtheadvantageofbeingfastertobeanswered.
ESSPRIwas originallydeveloped including10 questions, withthreeglobalscales(dryness,fatigueandpain),onescale
Table3–ResultsofcorrelationofESSPRIwithPGA,SSI,PROFAD,FACITandPROFAD-SSIinstruments.
ESSPRIresults PGAresults Mean±SD
Correlationcoefficient p-value Correlationcoefficient p-value
ESSPRI 1 0.000 0.602a 0.000 6.87± 1.97
PGA 0.602a 0.000 1 0.000 7.58± 2.15
SSI 0.444a 0.000 0.426a 0.000 18.19± 6.40
PROFAD 0.492a 0.000 0.498a 0.000 18.67± 6.23
FACIT 0.551a 0.000 0.455a 0.000 30.73± 11.35
PROFAD-SSI 0.527a 0.000 0.521a 0.000 4.61± 1.41
PGA,patient’sglobalassessment;FACIT,functionalassessmentofchronicillnesstherapy;PROFAD-SSI,profileoffatigueanddiscomfort–sicca symptomsinventory;SSI,SiccaSymptomsInventory;PROFAD,profileoffatigueanddiscomfort;ESSPRI,EULARSjögren’sSyndromePatient ReportedIndex;SD,standarddeviation.
onmentalfatigueand6scalesondryness(eye,mouth,vagina, skin,nose,airwaysandrespiratory).Inthemulticenter vali-dationstudy,itwasobservedthatthepsychometricproperties ofthethreeglobalscaleswereasgoodastheoverall instru-ment and,therefore, its final version was limited tothose 3globalscales.16 Thisstudy wasdevelopedinparallelwith
themulticenterstudyforESSPRIvalidation,andthereforethe completeinstrumentwasappliedandtested;andouranalysis areconsistent withinternationaldata.21 Thus, the
psycho-metricpropertiesofthefinalinstrumentwithonly3global scalesarecomparabletothepreliminaryinstrumentwith10 questions.However,the finalinstrument islessdetailed in relationtodrynessineachorgan.
Theconstruct validity ofthe original version of ESSPRI showed moderate correlation with PROFAD (r=0.73), SSI (r=0.66)andPGA(r=0.70),16andthesamewasobservedfor
the current version in the multicenter validation, r=0.68,
r=0.59 and r=0.70, respectively.21 Although correlation
coefficientsareslightlylower,thepresentstudyalsoshowed moderatecorrelationwiththesameinstruments.
ThecompositescoreofPROFAD-SSI,thoughnotvalidated initsoriginalversion,butvalidatedforthePortuguese Brazil-ian version,33 showed a moderate correlation (0.527) with
ESSPRI,avalueveryclosetothatfoundinthevalidationstudy oftheBrazilianversionofPROFAD-SSI(r=0.545).33
Thehigh reproducibilityfoundin thisstudy (0.911) was similar to that of the multicenter study (0.94).21 On the
otherhand,thelowinternalconsistencyoftheinstrument wasexpected,sincethethreedomainscharacterizedifferent aspectsofthediseaseanddonotalwaysconverge.
Thesampleconsistedofwomeninthe5thdecadeoflife, withtheirdiseaselasting7.2years,similartothedemographic profileofothercohorts6,34–38andtothatintheESSPRI
devel-opmentstudy.16
Duetothe loweducation levelandtothe illiteracyofa largepartoftheBrazilianpopulation,researchinhealthcare usuallygivesprioritytointerviewsingatheringinformation.28
Therefore, theauthors decidedbyachange inthe formof applicationoftheself-administeredquestionnaire,choosing aface-to-faceinterviewmodel.Thischangedoesnot invali-datetheuseofthisinstrumentbyself-administration,29and
thepsychometricequivalenceobtainedbetweentheoriginal andthe translatedversionconfirmed thesuitabilityofthis instrument.
Inoursample,43.5% ofanti-Roand/oranti-Lapositives were found, a slightly lower frequency than that in other
studies.6,16,34–37Despitethelowantibodyfrequency,the
diag-nosiswaswellestablished,asallpatientsunderwentsalivary biopsy and met the American-European criteria. The fre-quency ofbiopsies with a focus score ≥1 was 90.3%.This lower antibody frequency can be related to a less severe disease andcould partiallyexplainthe lowdiseaseactivity in most subjects in the sample. The antibody identifica-tion was carried out by double immunodiffusion method, which hasalowersensitivitythan other methods,suchas ELISA.Furthermore,thesamplecamefromaunitwherean activesearchisperformedforallcasesofdrynessand biop-siesaresystematicallyobtained.Forthisreason,manymild cases that otherwise would be underdiagnosed (antibody-negatives,without systemicmanifestations)are partofthe sample.
Varioustoolshavebeendevelopedandvalidatedtoassess the subjective characteristics indifferent autoimmune dis-eases and their implications in the quality oflife.39 In SS,
the symptoms offatigue, pain, and dryness40 might exert
high impact on the perception of illness and quality of life.
Interestingly,whilemostofthesamplestudiedwasmade upoflow-activitypatients,theESSPRIscorewashigh.This dis-sociationwasalreadydetectedinpreviousstudies,suggesting thatthepatient’ssymptomsandsystemiccomplicationsare twodifferentcomponentsofthedisease,reinforcingtheidea thatbothshouldbeevaluated,butseparately.21,41–44
Conclusion
ESSPRIevaluatesthepatient’s symptomswithSSandisan adaptable, reproducible and valid instrument for the Por-tugueselanguage,andcanbeusedintheBraziliancontext.
Funding
Financial support from Conselho Nacional de Desenvolvi-mentoCientíficoeTecnológico(CNPq).
Conflicts
of
interest
Annex
1.
Final
version
of
EULAR
Sjögren’s
Syndrome
Patients
Reported
Index
(ESSPRI).
Your doctor asked you to answer some questions related to your disease. To answer the your symptoms
of severity the consider please
questions, in the worst stages, only
during the last two weeks.
Please check the alternative that best describes your answer. Please answer all questions carefully.
Example:
No pain
0 1 2 3 4 5 6 7 8 9 10 Maximal imaginable
pain
1) How severe has your dryness been during the last 2 weeks?
No dryness
0 1 2 3 4 5 6 7 8 9 10
Maximal imaginable
dryness
2) How severe has your fatigue been during the last 2 weeks?
No fatigue
0 1 2 3 4 5 6 7 8 9 10
Maximum imaginable fatigue
3) How severe has your pain (joint or muscular pain, in your arms or legs) been during the last 2 weeks?
No pain
0 1 2 3 4 5 6 7 8 9 10
Maximal imaginable pain
r
e
f
e
r
e
n
c
e
s
1. ValimV,ZandonadeE,PereiraAM,deBritoFilhoOH,Serrano EV,MussoC,etal.PrevalênciadasíndromedeSjögren primáriaemimportanteáreametropolitananoBrasil.Rev BrasReumatol.2013;53:29–34.
2. AlamanosY,TsifetakiN,VoulgariPV,VenetsanopoulouAI, SiozosC,DrososAA.EpidemiologyofprimarySjögren’s syndromeinnorth-westGreece,1982–2003.Rheumatol(Oxf Engl).2006;45:187–91.
3. GøranssonLG,HaldorsenK,BrunJG,HarboeE,JonssonMV, SkarsteinK,etal.Thepointprevalenceofclinicallyrelevant primarySjögren’ssyndromeintwoNorwegiancounties. ScandJRheumatol.2011;40:221–4.
4. DelaleuN,JonssonR,KollerMM.Sjögren’ssyndrome.EurJ OralSci.2005;113:101–13.
5. VitaliC.ClassificationcriteriaforSjogren’ssyndrome:a revisedversionoftheEuropeancriteriaproposedbythe American-EuropeanConsensusGroup.AnnRheumDis. 2002;61:554–8.
6. SerorR,RavaudP,BowmanSJ,BaronG,TzioufasA,Theander E,etal.EULARSjogren’ssyndromediseaseactivityindex: developmentofaconsensussystemicdiseaseactivityindex forprimarySjogren’ssyndrome.AnnRheumDis.
2010;69:1103–9.
7. WesthoffG,DörnerT,ZinkA.Fatigueanddepressionpredict physicianvisitsandworkdisabilityinwomenwithprimary
Sjögren’ssyndrome:resultsfromacohortstudy.Rheumatol (OxfEngl).2012;51:262–9.
8.NgW-F,BowmanSJ.PrimarySjogren’ssyndrome:toodryand tootired.Rheumatol(OxfEngl).2010;49:844–53.
9.SutcliffeN,StollT,PykeS,IsenbergDA.Functionaldisability andendorgandamageinpatientswithsystemiclupus erythematosus(SLE),SLEandSjögren’ssyndrome(SS),and primarySS.JRheumatol.1998;25:63–8.
10.StrömbeckB,EkdahlC,ManthorpeR,WikströmI,JacobssonL. Health-relatedqualityoflifeinprimarySjögren’ssyndrome, rheumatoidarthritisandfibromyalgiacomparedtonormal populationdatausingSF-36.ScandJRheumatol.2000;29:20–8. 11.TensingEK,SolovievaSA,TervahartialaT,NordströmDC,
LaineM,NiissaloS,etal.Fatigueandhealthprofileinsicca syndromeofSjögren’sandnon-Sjögren’ssyndromeorigin. ClinExpRheumatol.2001;19:313–6.
12.BelenguerR,Ramos-CasalsM,Brito-ZerónP,DelPinoJ,Sentís J,AguilóS,etal.Influenceofclinicalandimmunological parametersonthehealth-relatedqualityoflifeofpatients withprimarySjögren’ssyndrome.ClinExpRheumatol. 2005;23:351–6.
13.ChampeyJ,CorrubleE,GottenbergJ-E,BuhlC,MeyerT, CaudmontC,etal.Qualityoflifeandpsychologicalstatusin patientswithprimarySjögren’ssyndromeandsicca symptomswithoutautoimmunefeatures.ArthritisRheum. 2006;55:451–7.
employmentanddisabilityinpatientswithSjogren’s syndrome.Rheumatol(OxfEngl).2009;48:1077–82. 15.SegalB,BowmanSJ,FoxPC,VivinoFB,MurukutlaN,
BrodschollJ,etal.PrimarySjögren’ssyndrome:health experiencesandpredictorsofhealthqualityamongpatients intheUnitedStates.HealthQualLifeOutcomes.2009;7:46. 16.SerorR,RavaudP,MarietteX,BootsmaH,TheanderE,Hansen
A,etal.EULARSjogren’sSyndromePatientReportedIndex (ESSPRI):developmentofaconsensuspatientindexfor primarySjogren’ssyndrome.AnnRheumDis.2011;70:968–72. 17.SerorR,TheanderE,BootsmaH,BowmanSJ,TzioufasA,
GottenbergJE,etal.OutcomemeasuresforprimarySjögren’s syndrome:acomprehensivereview.JAutoimmun.
2014;51:51–6.
18.BowmanSJ,BoothDa,PlattsRG.Measurementoffatigueand discomfortinprimarySjogren’ssyndromeusinganew questionnairetool.Rheumatol(OxfEngl).2004;43:758–64. 19.BowmanSJ,BoothDA,PlattsRG,FieldA,RostronJ.Validation
oftheSiccaSymptomsInventoryforclinicalstudiesof Sjögren’ssyndrome.JRheumatol.2003;30:1259–66. 20.BowmanSJ,HamburgerJ,RichardsA,BarryRJ,RauzS.
Patient-reportedoutcomesinprimarySjögren’ssyndrome: comparisonofthelongandshortversionsoftheProfileof FatigueandDiscomfort—SiccaSymptomsInventory. Rheumatology.2009;48:140–3.
21.SerorR,TheanderE,BrunJG,Ramos-CasalsM,ValimV, DornerT,etal.ValidationofEULARprimarySjögren’s syndromediseaseactivity(ESSDAI)andpatientindexes (ESSPRI).AnnRheumDis.2014.Availableat:http://ard. bmj.com/content/early/2014/03/11/annrheumdis-2013-204615 22.MeinersPM1,ArendsS,BrouwerE,SpijkervetFK,VissinkA,
BootsmaH.ResponsivenessofdiseaseactivityindicesESSPTI andESSDAIinpatientswithprimarySjögren’ssyndrome treatedwithrituximab.AnnRheumDis.2012;71:1297–302. 23.ChoHJ,YooJJ,YunCY,KangEH,LeeHJ,HyonJY,etal.The
EULARSjogren’ssyndromepatientreportedindexasan independentdeterminantofhealth-relatedqualityoflifein primarySjogren’ssyndromepatients:incomparisonwith non-Sjogren’ssiccapatients.Rheumatol(Oxf).
2013;52:2208–17.
24.HackettKL,NewtonJL,FrithJ,ElliottC,LendremD,FoggoH, etal.ImpairedfunctionalstatusinprimarySjogren’s syndrome.ArthritisCareRes(Hoboken).2012;64:1760e4. 25.NgW-F,MitchellS,LendremD,BowmanSJ,PriceE,PeaseC,
etal.HowgoodaretheEULARSjögren’ssyndromedisease activityindex(ESSDAI),andEularSjögren’ssyndrome patientsreportedindex(ESSPRI)inpredictinghealthstatusin primarySjögren’ssyndrome?AnnRheumDis.2012;71:553. 26.HerdmanM,Fox-RushbyJ,BadiaX.Amodelofequivalencein
theculturaladaptationofHRQoLinstruments:the universalistapproach.QualLifeRes.1998;7:323–35. 27.HasselmannMH,ReichenheimME.Adaptac¸ãotranscultural
daversãoemportuguêsdaConflictTacticsScalesFormR (CTS-1),usadaparaaferirviolêncianocasal:equivalências semânticaedemensurac¸ão.CadSaúdePública.
2003;19:1083–93.
28.MoraesCL,HasselmannMH,ReichenheimME.
Portuguese-languagecross-culturaladaptationoftheRevised ConflictTacticsScales(CTS2),aninstrumentusedtoidentify violenceincouples.CadSaúdePública.2002;18:163–76. 29.ReichenheimME,MoraesCL.Operationalizingthe
cross-culturaladaptationofepidemiologicalmeasurement instruments.RevSaúdePública.2007;41:665–73.
30.ReichenheimME,PaixãoCMJr,MoraesCL.Adaptac¸ão transculturalparaoportuguês(Brasil)doinstrumento Hwalek-SengstockElderAbuseScreeningTest(H-S/EAST)
usadoparaidentificarriscodeviolênciacontraoidoso.Cad SaúdePública.2008;24:1801–13.
31.HairJFJr.Factoranalysis.Multivariatedataanalysis.4thed. NewJersey:PrenticeHall;1995.
32.SerranoEV,ValimV,MiyamotoST,AltoeR,PaganottiMA, CadeNV.Adaptac¸ãotransculturaldo“EULARSjögren’s SyndromeDiseaseActivityIndex(ESSDAI)”paraalíngua portuguesa.RevBrasReumatol.2013;53:483–93.
33.MiyamotoST,PaganottiMA,SerranoEV,GiovelliRA,ValimV. Avaliac¸ãodafadigaedasecuranasíndromedeSjögren Primária:versãobrasileiradoProfileofFatigueand Discomfort–SiccaSymptomsInventory(shortform) (Profad-SSI-SF)”.RevBrasReumatol.2015;55:113–22. 34.BowmanSJ,SutcliffeN,IsenbergDA,GoldblattF,AdlerM,
PriceE,etal.Sjögren’sSystemicClinicalActivityIndex(SCAI) –asystemicdiseaseactivitymeasureforuseinclinicaltrials inprimarySjögren’ssyndrome.Rheumatol(OxfEngl). 2007;46:1845–51.
35.VitaliC,PalombiG,BaldiniC,BenucciM,BombardieriS, CovelliM,etal.Sjögren’sSyndromeDiseaseDamageIndex anddiseaseactivityindex:scoringsystemsforthe assessmentofdiseasedamageanddiseaseactivityin Sjögren’ssyndrome,derivedfromananalysisofacohortof Italianpatients.ArthritisRheum.2007;56:2223–31.
36.MeinersPM,ArendsS,BrouwerE,SpijkervetFKL,VissinkA, BootsmaH.ResponsivenessofdiseaseactivityindicesESSPRI andESSDAIinpatientswithprimarySjögren’ssyndrome treatedwithrituximab.AnnRheumDis.2012;71:1297–302. 37.SerorR,MarietteX,BowmanS,BaronG,GottenbergJE,
BoostmaH,etal.Accuratedetectionofchangesindisease activityinprimarySjögren’ssyndromebytheEuropean LeagueAgainstRheumatismSjögren’sSyndromeDisease ActivityIndex.ArthritisCareRes.2010;62:551–8.
38.RisseladaAP,KruizeAA,BijlsmaJWJ.Clinicalapplicabilityof theEULARSjogren’ssyndromediseaseactivityindex:a cumulativeESSDAIscoreaddsindescribingdiseaseseverity. AnnRheumDis.2012;71:631.
39.CamparA,IsenbergDA.PrimarySjögren’ssyndromeactivity anddamageindicescomparison.EurJClinInvest.
2010;40:636–44.
40.MarietteX,RavaudP,SteinfeldS,BaronG,GoetzJ,HachullaE, etal.InefficacyofinfliximabinprimarySjögren’ssyndrome: resultsoftherandomized,controlledTrialofRemicadein PrimarySjögren’sSyndrome(TRIPSS).ArthritisRheum. 2004;50:1270–6.
41.NgW-F,BowmanS,GriffithsB,MitchellS,PriceE,PeaseC, etal.Relationshipbetweendiseaseactivityofprimary Sjogren’ssyndromeandPatientreportedoutcomeedata fromaninterimanalysisoftheUKprimarySjögren’s syndromeregistry.AnnRheumDis.2011;70:510.
42.SerorR,GottenbergJ-E,Devauchelle-PensecV,DubostJJ,Le GuernV,DieudéP,etal.Assessmentofsystemicdisease activityiscomplementarytoassessmentofpatient’s symptomsinprimarySjogren’ssyndrome.AnnRheumDis. 2011;70:505.
43.SerorR,RavaudP,BowmanSJ,BootsmaH,TheanderE,Vitali C,etal.Patients’complaintsdependonsystemicstatusin patientwithprimarySjögren’ssyndrome.AnnRheumDis. 2010;69:569.
44.SerorR,GottenbergJE,Devauchelle-PensecV,DubostJJ,Le GuernV,HayemG,etal.EuropeanLeagueAgainst