www.jped.com.br
ORIGINAL
ARTICLE
Analysis
of
analgesic,
antipyretic,
and
nonsteroidal
anti-inflammatory
drug
use
in
pediatric
prescriptions
夽
Tânia
R.
Ferreira,
Luciane
C.
Lopes
∗UniversidadedeSorocaba(UNISO),Sorocaba,SP,Brazil
Received12December2014;accepted15April2015 Availableonline9October2015
KEYWORDS
Antipyretics; Nonsteroidal anti-inflammatory drugs;
Prescriptiondrugs; Pediatrics; Analgesics
Abstract
Objective: Data on clinicalpractice inpediatrics onthe use ofanalgesic, antipyretic, and
nonsteroidalanti-inflammatorydrugsconsideringthebestavailableevidenceand regulatory-agencyapproveduseareuncertain.Thisstudyaimedtodeterminethefrequencyofprescription ofthesedrugsaccordingtothebestscientificevidenceanduseapprovedbyregulatoryagencies.
Methods: Thiswasacross-sectionalstudyof150pediatricprescriptionscontaininganalgesic,
antipyretic,andnonsteroidalanti-inflammatorydrugs,followedbyinterviewwithcaregiversat 18locations(nineprivatedrugstoresandnineBasicHealthUnitsoftheBrazilianUnifiedHealth System).Theassessedoutcomesincludedrecommendeduseorusewithnocontraindication, indications withbenefit evidence,andhealth surveillance agency-approveduse.Data were analyzedinelectronicdatabasesandthevariablesweresummarizedbysimplefrequency.
Results: Atotalof164analgesic,antipyretic,andnonsteroidalanti-inflammatorydrugswere
prescribedto150childrenaged1---4years(38.6%).Dipyrone wasincludedin82(54.6%)and ibuprofenin40(26.6%)prescriptions.Non-recommendeduseswereidentifiedin15%of pre-scriptionsandcontraindicateduseswereobservedin13.3%.Nimesulide(1.5%)isstillprescribed tochildrenyoungerthan12years.Thedosewasincorrectin74.3%ofprescriptions contain-ingdipyrone.Ofthe211reportedclinicalindications,56(26.5%)hadnoevidenceofbenefit accordingtothebestavailablescientificevidenceand66(31.3%)hadindicationsnotapproved bytheregulatoryagencies.
Conclusion: Therearesignificantdiscrepanciesbetweenclinical practiceandrecommended
useofanalgesic,antipyretic,andnonsteroidalanti-inflammatorydrugsinpediatrics.
©2015SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
夽
Pleasecitethisarticleas:FerreiraTR,Lopes LC.Analysisofanalgesic,antipyretic,andnonsteroidalanti-inflammatorydrugusein
pediatricprescriptions.JPediatr(RioJ).2016;92:81---7.
∗Correspondingauthor.
E-mail:luslopes@terra.com.br(L.C.Lopes). http://dx.doi.org/10.1016/j.jped.2015.04.007
PALAVRAS-CHAVE
Antipiréticos; Anti-inflamatórios nãoesteroides; Prescric¸ãode medicamentos; Pediatria; Analgésicos
Análisedousodeanalgésicos,antipiréticoseanti-inflamatóriosnãoesteroides emprescric¸ãopediátrica
Resumo
Objetivo: Dados sobrea práticaclínica em pediatrianouso deanalgésicos, antipiréticose
anti-inflamatóriosnãoesteroidesconsiderandoamelhorevidênciadisponíveleusoaprovado poragênciasreguladorassãoincertos.Esteestudotemcomoobjetivoverificarafrequênciade prescric¸ãodetaismedicamentossegundoamelhorevidênciacientíficaeousoaprovadopor agênciasreguladoras.
Método: Estudotransversalde150prescric¸õespediátricas,contendoanalgésicos,antipiréticos
eanti-inflamatóriosnão esteroides,seguidodeentrevistaaoscuidadores,emdezoito locais (novedrogariasprivadasenoveUnidadesdeSaúdedoSUS).Osdesfechosavaliadosincluíram usorecomendadoousemcontraindicac¸ão,indicac¸õescomevidênciadebenefícioeouso autor-izadoporagênciasdevigilânciasanitária.Osdadosforamanalisadosembancoeletrônicoeas variáveissumarizadasporfrequênciasimples.
Resultados: Foramprescritos164analgésicos,antipiréticoseanti-inflamatóriosnãoesteroides
para as 150 crianc¸as comidade entre 1e 4 anos(38,6%). Dipirona constou em 82 (54,6%) eibuprofenoem40(26,6%).Usosnãorecomendadosforamencontradosem15%dasreceitas e usoscontraindicadosem 13,3%.Nimesulida(1,5%)aindaéutilizada emcrianc¸ascommenos de 12 anos. Em 74,3% das prescric¸ões contendo dipirona a dose estava incorreta. Das 211 indicac¸ões clínicas referidas 56 (26,5%)não tinham evidências de benefício segundo a melhorprovacientíficadisponível,66(31,3%)eramindicac¸õesnãoaprovadasemagênciasde vigilânciasanitária.
Conclusão: Existemimportantesdiscrepânciasentrepráticaclínicaerecomendac¸õesdeusode
analgésicos,antipiréticoseanti-inflamatóriosnãoesteroidesempediatria.
©2015SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos reservados.
Introduction
In Brazil, as in other developing countries, regulatory policies and regulations on the sales and prescription of medicationsforthepediatricagerangearestillinsufficient forthesectortobefreeofrisksrelatedtoinadequatedrug prescriptionsanduses.
Analgesics, antipyretics and nonsteroidal
anti-inflammatorydrugs(NSAIDs)arethemostoftenprescribed medications in the pediatric age group.1 Predominantly
naproxen,ketoprofen, andibuprofen,whichare over-the-counter(OTC)medicationsregulatedbyRDCNo.138/2003.2
Nimesulideandotherdrugsofthesamegroup,althoughnot includedintheOTClist,canbepurchasedinanypharmacy inBrazilwithoutaprescription.
Although these drugs have potential adverse effects, they are widely sold in pharmacies, disregarding restric-tionsofuse,indications,toxicity,andcontraindicateddrug interactions.They areoften prescribedwithout adefined therapeuticgoal,generatingunnecessarycosts.
Formild tomoderatepain,ingeneral,analgesics with-outanti-inflammatoryeffect(low-doseacetylsalicylicacid andibuprofen,orparacetamol)shouldbeprescribed.NSAIDs have similar efficacy, but their selection should consider relativetoxicity, cost,and approvedage group (based on safetyand efficacy studies for the drug).NSAIDs have an ‘‘all or nothing’’ effect, i.e., increasing the dose does notincrease therapeutic efficacy, butresults in increased adverseeffects.3
Althoughfeverisabeneficialresponseinmostcases,it isanimportantcauseofanxietyforparentsandphysicians. Thesearchformoreefficienttreatmentshasledtotheuse ofantipyreticcombinationsinpediatrics,muchappreciated bycaregiversandhealthcareprofessionals,butwhose effi-cacyhasbeentestedforonlyafewyearsinclinicaltrials.4---6
Thenewschemesconsistofcombinationsofibuprofenand paracetamoladministeredatvaryingtimes.Themain con-cernwiththesetreatmentsissafety,astheymayincrease the risk of kidney toxicity and Streptococcus infection.7,8
Therefore,itisnotknownwhetherthesecombinationsare moreeffectivethanandassafeasmonotherapyinchildren withfever.6
In developed countries, the indication of analgesics, antipyretics, andNSAIDs inpediatricpatientsis extremely limited.Currently,onlytwodrugsareapprovedbythe Euro-peanMedicineAgency(EMEA)forthetreatmentoffeverin children:paracetamolandibuprofen.9Millionsofeuroshave
beenspenttoraiseawarenessamongprescribersregarding therationaluseofdrugs,seekingtomodifyinadequate pre-scriptioncriteriaandhabits.10
Drug prescription is a legal document, for which the person prescribing the drug (physician) and the person dispensing it (pharmacist) are responsible and subject to sanitarycontrolandsurveillancelegislation.
frequenciesfor whichithasnotbeenapproved,thus con-figuring ‘‘offlabel’’use. This situationmaycontribute to children’sexposuretoadverseevents,mainlydueto inade-quatedruguse.1
Dataonclinicalpracticeinpediatricsregardingtheuseof thesedrugs,consideringthebestavailableevidenceanduse approvedbyregulatoryagenciesareuncertain.Therefore, thisstudyaimedtoverifythefrequencyofprescriptionof analgesics,antipyretics, andNSAIDs according tothebest scientificevidenceanduseapprovedbyregulatoryagencies.
Methods
Studydesign,locationandperiod
This wasa cross-sectional study,based onthe analysisof pediatric prescriptions and information providedby care-givers.
Theauthorschosetoperformanexploratory,descriptive study aiming toidentify, record, and analyze the charac-teristicstogenerateahypothesisaboutthecriteriausedin pediatricprescriptionofanalgesic,antipyretic,andNSAIDs. Althoughthissubjectcanbeaconstanttargetofdebate,it hasnotbeenexploredindepth.
The study was initiated after approval by the Ethics andResearchCommittee,UniversidadedeSorocaba(UNISO) (DocumentNo.037/08,11/19/2008).
Selectionofthestudysites,criteriaandcase managementprocedures
Datacollectionwascarriedoutinnineprivatepharmacies andnineBasicHealthUnits(BHUs)fromtheBrazilian Uni-fiedHealthSysteminthemunicipalityofSorocaba,SP,which were chosen by drawinglots, considering their geograph-ical location. The field research was conducted for nine months.Volunteers(caregiverswhohadapediatric prescrip-tion)wererecruited toparticipateinthe studyaccording toorderofarrivalatthepharmacy.Theresearchwas con-ductedonceaweekatdifferenthours.Thisstudyusedtwo data sources: pediatric prescriptions and interviews with caregiverswhohadtheprescriptions.Detailsoneligibility criteria,datacollection,interviews,andthequestionnaire usedhavebeenpreviouslypublishedbyFerreiraetal.11
Indicationclassificationaccordingtothebest availableevidenceandapprovalofregulatory agencies
Fortheindicationclassificationaccordingtothebest avail-able clinical evidence of efficacy, theoretical data from Dynamed® (EBSCO, MA, USA),12 Clinical Evidence,13 and
Drugdex®SystemThomsonMicromedex14wereused.To
ver-ifytheapprovedindications,drugregistrationdatafromthe Brazilian HealthSurveillanceAgency(Agência Nacionalde VigilânciaSanitária---ANVISA)andfromtheFoodandDrug Administration(FDA)wereused.
Drug indication wasclassified according to recommen-dation of use: use is not recommended (i.e., it can be usedwithprecautions)andcontraindicateduse(absolutely
prevents the use). Information on patient characteris-tics (age, comorbidity, among others) and the diagnosis reported by the caregiver were verified considering the recommendedinformationonusefoundinthedatabases.
Dataanalysis
Continuousvariablesweredescribedbymeansandstandard
deviations or median, minimum, and maximum values,
as appropriate, whereas binary variables were described by proportions. A descriptive exploratory analysis was employed.
Thereportedindicationswereclassifiedas:(i)thosewith definedscientificevidence;(ii)thosewithno contraindica-tionsforuse;(iii)thoseapprovedbyaregulatoryagency;or (iv)thosewithouttheseproperties.
Results
SamplecompositionisdescribedinFig.1.
Sample characteristics were described in the study by Ferreiraetal.11Therewasahigherprevalenceofthreeor
moredrugs perprescription,found intheagegroupof 1---4years(interquartilerange,3.5---8.7),and60%ofthe pre-scriptionsfailedtomentionthemedicalspecialty.In51.3% of cases, the mothers were the caregivers who took the prescriptiontobefilled.
The 150 patients weretaking 506 drugs, of which 431 (85.2%)wereprescribed.However,caregiversof58children reportedthat they were alsousing other medications, of which75(14.8%)werenotincludedintheanalyzed prescrip-tionandwerethereforetheresultofotherprescriptionsor self-medication.Ninety-onepatientsdidnotuseanydrugs otherthanthoselistedintheassessedprescription(datanot shown).
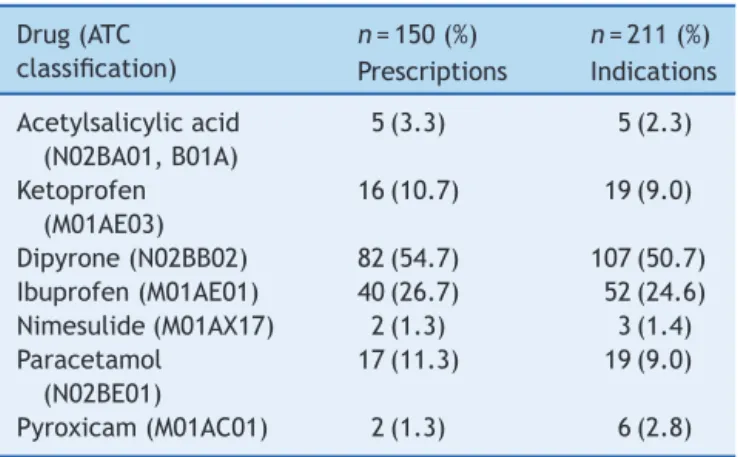
Inthe150prescriptions,thesevenanalgesics, antipyret-ics, and NSAIDs identified were prescribed 164 times for 211indications. This meanstherewereprescriptions with more than one drug from this group. The most com-monly observed drug was dipyrone in 82 cases (54.7%), followed by ibuprofen, in 40 (26.7%; Table 1). Accord-ingtothe InternationalClassification of Diseases (ICD10) the most frequent clinical indications for dipyrone, in 68
Table1 Reportedclinicalindications.
Drug(ATC classification)
n=150(%) n=211(%)
Prescriptions Indications
Acetylsalicylicacid (N02BA01,B01A)
5(3.3) 5(2.3)
Ketoprofen (M01AE03)
16(10.7) 19(9.0)
Dipyrone(N02BB02) 82(54.7) 107(50.7) Ibuprofen(M01AE01) 40(26.7) 52(24.6) Nimesulide(M01AX17) 2(1.3) 3(1.4) Paracetamol
(N02BE01)
17(11.3) 19(9.0)
Pyroxicam(M01AC01) 2(1.3) 6(2.8)
Prescriptions of potentially eligible pediatricv patients (n=245)
Pediatric prescriptions and interviews with caregivers
included (n=150)
Excluded (n=95)
Not the child's parents/tutors or did not go to the medical consultation (n=90)
Did not agree to participate in the study (n=5)
N-SUS (n=49)
SUS (n=101)
Figure1 Flowchartofsamplecomposition.N-SUS,non-SUS;SUS,BrazilianUnifiedHealthSystem.
cases,(63.5%)werefor thetreatment ofsymptomsofflu, colds,influenza-related infections, tonsillitis, pharyngitis, andother respiratory diseases (J00-J11.9). The most fre-quentindicationsforibuprofen,in17cases,(32.7%)wereto treatunspecifiedsymptomsandsigns,suchaspain,fever, headache,andothers(R50-R52).
Table2showsthenon-recommendedandcontraindicated useofanalgesics,antipyretics,andNSAIDs.Inthissample, six(2.84%)caseswereindicationstotreatsymptoms asso-ciatedwithallergic conditions(asthma or rhinitis), which isnotrecommendedduetothepossibilityofdisease exac-erbation.Additionally,nimesulide(M01AX17)andpiroxicam (M01AC01)wereindicatedfor painandfevermanagement inchildrenyoungerthan12years.
Table3showstheindicationswithnoevidenceof bene-fitaccordingtothebestscientificevidenceandthenumber ofindications not approved byANVISAor theFDA. It was observedthat100%ofthereportedindicationsfor acetylsal-icylicacid(N02BA01,B01A),dipyrone(N02BB02),nimesulide
(M01AX17),andpiroxicam(M01AC01)hadnoclinicalstudies tosupporttheiruse.
Acetylsalicylic acid had five indications: four for the treatment of sickle cell anemia and one for tonsillitis, whose prescription curiously stated that a tablet should be diluted in half a glass of water for gargling every 8h for 5 days.Theseinstructions arenotapproved byhealth agenciesandhavenorecommendedusebasedonscientific evidence.
Inthis sample, dipyronewasprescribed to82 patients (54.6%) and was combined with other analgesics and antipyretics(AA)orNSAIDsineight(9.7%)cases.Thedoses werehigherthanthoserecommendedbyregulatory agen-cies or eventhe drug leafletsin 41(55.4%) prescriptions. Manyof themincluded arecommendeduse thatdoes not appearinanyofficialprotocolorconsulteddatabase.
Ibuprofenwasprescribedfor52clinicalconditions;in19 (36.5%),itsuseisnotbasedinscientificevidenceor autho-rizedbyanyhealthagency.Itisnoteworthythattherewere
Table2 Characterizationofnon-recommendeduse(usewithcaution)andcontraindicateduseofAAandNSAIDsfoundinthe prescriptions,consideringpatientcharacteristicsandclinicalindication(reporteddiagnosis).
Typeofrecommendation Drug Typeofindication Rationalea
Non-recommendeduse (usewithcaution)n=11 (5.2%)
Dipyrone(N02BB02) Sicklecellanemia(n=1) Canintensifythecrises
Ketoprofen(M01AE03) Asthma(n=1) Canintensifythecrises Bronchitis(n=2) Canintensifythecrises Ibuprofen(M01AE01) Asthma(n=3) Canintensifytheasthmacrises
Allergicrhinitis(n=2) Canintensifythebronchitiscrises Esophagealreflux(n=1) Canintensifythebronchitiscrises Paracetamol(N02BE01) Bronchitis(n=1) Canintensifythebronchitiscrises
Contraindicatedusen=5 (2.4%)
Pyroxicam(M01AC01) Influenza(n=1) Contraindicatedfortheagegroup Headache(n=1) Contraindicatedfortheagegroup Acutecough(n=1) Contraindicatedfortheagegroup Nimesulide(M01AX17) Tonsillitis(n=1) Contraindicatedfortheagegroup Fever(n=1) Contraindicatedfortheagegroup
AA,analgesicandantipyretic;NSAIDs,non-steroidalanti-inflammatorydrugs.
aAccording to Dynamed (https://dynamed.ebscohost.com), Clinical Evidence (http://clinicalevidence.bmj.com/x/index.html),
Table3 Frequencyofreportedindications,withnoscientificevidenceofbenefitandusenotapprovedbyhealthagencies.
Drugname ATC Totalof
indications
Indicationswith noevidence
n(%)
Indicationsnotapproved byhealthagencies
ANVISA FDA
n(%) n(%)
Acetylsalicylicacid N02BA01 5(2.3) 5(100) 5(100) 5(100)
Ketoprofen M01AE03 19(9.0) 4(21.0) 4(21.0) 19(100)
Dipyrone N02BB02 107(50.7) 15(14.0) 15(14.0) 107(100)
Ibuprofen M01AE01 52(24.6) 19(36.5) 19(36.5) 19(36.5)
Nimesulide M01AX17 3(1.4) 3(100) 3(100) 3(100)
Paracetamol N02BE01 19(9.0) 4(21.0) 4(21.0) 4(21.0)
Pyroxicam M01AC01 6(2.8) 6(100) 6(100) 6(100)
ATC,AnatomicalTherapeuticChemicalCode;ANVISA,BrazilianHealthSurveillanceAgency;FDA,FoodandDrugAdministration.
indicationsfor patientswithbronchitis,stomatitis,reflux, rhinitis-sinusitis,andcough.
Paracetamolwasindicatedfor19clinicalconditions,four ofwhich(21%)withoutscientificevidence,including respi-ratoryallergies,reflux,cough,andstomatitis.
Discussion
Mainfindings
The prescriptions (150) containing the seven analgesic, antipyretic,NSAIDs (acetylsalicylicacid,ketoprofen, dipy-rone,ibuprofen, nimesulide,paracetamol,andpiroxicam) forpediatric usehad56(26.5%)indicationswithno scien-tificevidence. Of the 211 reportedindications, 14 (6.6%) were not authorized by any regulatory agency, 11 (5.2%) werenotrecommendedorshouldbeusedwithcaution,and five(2.4%)hadcontraindicateduse.
Among thesesevendrugsthathad100%indications not approvedbytheFDAorANVISA,mainlybecausetheywere prescribedtochildrenyoungerthan12years,are ketopro-fen(approvedbyANVISA, butnotbytheFDA),nimesulide (approvedby ANVISA,but notbythe FDA),andpiroxicam (notapprovedbyeitheragency).Dipyroneisnotapproved forusebytheFDA.
Comparisonwithotherstudies
Inthepresentstudy,thehighestprevalenceofuseof anal-gesic,antipyretic,NSAIDswithnoevidenceofbenefitwas foundfordrugsprescribedtochildrenyoungerthanthe
rec-ommendedage.Several drugswhose usewasapprovedin
Brazilin2009(ketoprofen,nimesulide,andpiroxicam)have userestrictionsaccordingtoage,asspecifiedbyregulatory agenciesinothercountries.
It is noteworthy the case of nimesulide, which was
never approved for pediatric use and whose sales have
been suspended in several countries (Ireland, England, Australia, France, Finland, Portugal, and Spain)15 due to
the possibility of liver damage, skin reactions, and fatal Reye’ssyndrome;itwasinitiallyapprovedforpediatricand adultuseinBrazil.Theapprovalofnimesulideforpediatric useinBrazilbefore2007,withoutmoreconclusivestudies regardingitssafetyinthispopulation,14wassurprisingand
canexplaintheinadequateprescriptionsofthismedication still observed in the present sample. Currently, ANVISA requiresleaflets toinclude:‘‘This product is notsuitable forchildrenyoungerthan12years.’’
However,whenaccessingdrugsalessites16itispossibleto
identifyintheBrazilianmarketatleast16laboratoriesthat manufacturenimesulideasoralsolutionatconcentrationsof 10mg/mL,50mgmL,or100mg/mL,whosedrugleafletsstill bringdoserecommendationforchildrenaged>1yearoldof 1drop/kgforthetreatmentofpainandinjuries.Conversely, when the same query is carried out in ANVISAelectronic druginformationsite,onlyfourlaboratorieshaveregistered thedrugleaflet of nimesulideasoral solution,where the followingorientationscanbe found:‘‘Adult andpediatric useinchildren olderthan12 years’’and‘‘This productis contraindicatedforchildrenyoungerthan12years.’’17
This discrepancy in information can confound pre-scribers,healthcareprofessionals,andconsumers, increas-ingtherisksofinappropriateuseofthismedicationbythe pediatricpopulation.
TheWorldHealthOrganizationhastwiceissuedwarnings againstthemarketingofnimesulide;in2003,itwasplaced in the category of special products under pharmacovigi-lance. In2007, the EMEA started asystematic analysis of liverdamagecausedbythisproductanddecidedto main-tainitinthemarket,withitsuseapprovedforchildrenolder than 12 years,providing they areunder constant surveil-lanceandlimitingtheusetoamaximumof15consecutive days.18
A similar situation was observed with piroxicam in Brazil. Until 2009, drug presentations included oral solu-tion/dropswithoutage-restricteduse.Currently,piroxicam isnolongerfoundinthispharmaceuticalformand presen-tation,anditsindicationisrestrictedtochildrenolderthan 12years.
As for the ketoprofen, it appears that it is sold in Brazil in the pediatric formulation as a medication with analgesicand antipyreticproperties at lowdoses, aswell asanti-inflammatorypropertiesat largerdoses, indicated forthesymptomaticreliefoffeverand/orpaininchildren older than 6 months. The leaflet contains directions for use in specialpopulations, that is, children younger than 6months,inwhomdrugsafetyandefficacyhavenotbeen establishedyet.19 TheFDAdoes notapprove itsuseinthe
of fever (Class IIb, category B), osteoarthritis (class IIb, category C), pain (class IIa, category B) and rheumatoid arthritis(classIIb,categoryC).13
Ufer etal. confirmed the association between the use of drugs not approved for pediatric use and the preva-lenceofadverseeffects.1Wiltonetal.observedthat20%of
pediatricprescriptionsinSwedencontaineddrugsrecently introducedinthemarket,includingapercentageofdrugs with some contraindication for the age range.20 In this
clinical---epidemiological scenario, it is believed that the numberofmedicationsconsidered inappropriatefor pedi-atricuseishigherthanthatdisclosedbyseveralstudies.21,22
In 10% of the present sample, twoAA or NSAIDs were includedin thesame prescription,tobeusedalternately. This indication lacks evidence, which increases the risk of liver damageand may create doubts for the caregiver about administration intervals.23,24 A study in Argentina,
with 1600 pediatricians, showed that 59% of them alter-natetwoantipyretics, andconcludedthat thispracticeis morecommonamongphysicianswithlessexperience.25 It
isoddthatsomeclinicalprotocolsoftheBrazilianMinistry ofHealthindicatethispractice;onenoteworthyexampleis thetreatmentofdenguefever.
Consideringtheuncertainty surroundingthesuperiority orsafetyofcombinedantipyreticregimenswhencompared withmonotherapy,paracetamolor ibuprofenaloneshould continuetobeused.NationalInstituteofHealthand Clini-calExcellence(NICE)guidelinesstatethatparacetamoland ibuprofen should not be routinely administered together orusedinterchangeably. However,ifthepatientdoes not respondtooneofthesedrugs,analternativedrugcouldbe used.26
Another interesting finding in the present sample was relatedtotheprescribeddose.Dipyrone,thedrugwiththe highestprevalenceofprescriptionsatinadequatedoses,had 55.4%ofprescriptionsabovetherecommendedorapproved doses.Thesedataaredifferentfromthoseobtainedby Fer-reiraet al.,27 whoobserved dipyroneuse at lower doses
administeredby routesnot indicated tochildren younger than1year.Alvesetal.28 observedthatchildrenreceived
doseshigherthanthoserecommendedbythedrugleaflet, increasingthe risksof adverse events, including hypoten-sion.
Thecalculationofthepediatricdoseisstillamajor ther-apeutic problem.Dose calculation based onthe patient’s ageisnotalwaysthebestoption,especiallyininfants,and canresultinoverdose.Patientsofthesameagemaydifferin bodymass29;however,calculatingthepediatricdosebased
onbodyweightofthesubjectisnotindicatedeither,asit isknownthatchildren’smaturationprocessoccurgradually anddoesnotcorrespondtotheindividualgaininstature.30
Studieswiththisage grouparestillnecessarytoestablish theoptimaldose.Thisfactmayexplaintheobserveddose variationsinthissample.
Studystrengthsandlimitations
Thisstudypresentedthefirstdetaileddataonuseof anal-gesic,antipyretic,andNSAIDsinpediatricpatientsinBrazil. Patients were included shortly after the medical consul-tation,which reduced theconfoundingfactor of recalling
thereportedindicationsorthechild’ssignsandsymptoms. Patients were identified throughout the year, in all four seasons,reducingpossibleseasonalitybiases.Additionally, concerned about the population representativeness, the authorsselected 18differentlocations, includingpatients treatedatthepublicandtheprivatesectors.
Adetailedinterviewscriptwasusedbytwotrained inter-viewers,andtheanswerswerecross-checkedwiththedata containedintheprescriptions.Perhapsthemainlimitation ofthisstudywasitssamplesize,butregardingthisaspect, adescriptiveexploratoryanalysiswaschosen,withno asso-ciationsbetweenvariables.
Sourcesofevidence recommendedby regulatory agen-cies and by the World Health Organization were usedfor dataanalysis(Dynamed,ClinicalEvidence,Drugdex®System
ThomsonMicromedex).12---14
Practicalimplicationsandfinalconsiderations
Drugprescriptionshouldbebasedonthebestavailable evi-denceofbenefitandonthevaluesandpreferencesofthe individualthatwillbetreated.
Addressingtheparents’anxietyandfearsaboutfeverand educating them onthe immunological usefulnessof fever and the risks associated with the overuse of antipyretics shouldremainapriority.
Thereisurgent needfor interventionmeasures indrug dispensing, which will result in the rational use of these drugs, as well as a positive impact on health outcomes. Drugregistration policiesthat considerthe bestavailable scientificevidencecoulddecreasetheadventofdrugswith unclearuseindications.
These findings show that important differences can
be observed between clinical practice in pediatrics
regarding the use of AA and NSAIDs and
recommenda-tionsbasedonthebestavailablescientificevidenceanduse approvedbyregulatoryagencies.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.UferM,RaneA,KarlssonA,KimlandE,BergmanU.Widespread off-label prescribing of topical but not systemic drugs for 350,000paediatricoutpatientsinStockholm.EurJClin Pharma-col.2003;58:779---83.
2.Brasil. Ministério da Saúde Agência Nacional de Vigilância
Sanitária (Anvisa). Resoluc¸ão RDC n◦ 138, de 29 de maio
de 2003. Dispõe sobre o enquadramento na categoria de
venda de medicamentos. Brasília, DF: Diário Oficial da
União,PoderExecutivo;2003[cited28.03.15].Availablefrom
http://www.fitoterapia.com.br/portal/images/resolucao138.pdf 3.PinheiroRM,WannmacherL.Usoracionaldeanti-inflamatórios
não esteroides. Temas selecionados. 2010;5:1---15 [cited 28.03.15]. Available from http://www.cff.org.br/cebrim/ arquivo/7360/201203161748130.pdf
5.HayAD,CostelloeC,RedmondNM,MontgomeryAA,FletcherM, HollinghurstS,etal.Paracetamolplusibuprofenforthe treat-mentoffeverinchildren(PITCH):randomizedcontrolledtrial. BMJ.2008;337:a1302.
6.SarrellEM,Wielunsky E,Cohen HE.Antipyretictreatmentin youngchildrenwithfever:acetaminophen,ibuprofen,orboth alternatinginarandomized,double-blindstudy.ArchPediatr AdolescMed.2006;160:197---202.
7.Eguia L, Materson B. Acetaminophen-related acute renal failure without fulminant liver failure. Pharmacotherapy. 1997;17:363---70.
8.Lesko SM, O’Brian KL, Schwartz B, Vezina R, Mitchell AA. InvasivegroupAstreptococcalinfectionandnonsteroidal anti-inflammatorydruguseamongchildrenwithprimaryvaricella. Pediatrics.2001;107:1108---15.
9.EuropeanMedicinesAgencyHumanMedicine.Ibuprofen, para-cetamol;2009 [cited29.11.14]. Available from http://www. ema.europa.eu/docs/enGB/documentlibrary/PIPdecision/ WC500005655.pdf
10.Nascimento ÁC. Propaganda de medicamentos. É possível regular? [thesis]. Rio de Janeiro: Universidade do Estado doRio de Janeiro, Instituto de Medicina Social;2007 [cited 28.03.14]. Available from http://bvsms.saude.gov.br/bvs/ premiomedica/pdfs/trabalhos/mencoes/alvaronascimento trabalhocompleto.pdf
11.Ferreira TR, Barberato Filho S, Borgatto AF, Lopes L.C. Analgésicos,antipiréticos eanti-inflamatórios nãoesteroides em prescric¸ões pediátricas. Cien Saude Colet (Rio J). 2013;18:3695---704.
12.DynaMed Editorial Team. [cited 07.12.14]. Available from http://www.dynamed.com/home/.
13.Drugdex® System Thomson Micromedex. In: Klasco RK, edi-tor.DRUGDEXsystem.GreenwoodVillage,Colorado:Thomson Micromedex; 1974---2010 [cited 07.12.14]. Available from http://www.periodicos.caps.gov.br
14.Clinical Evidence. [cited 07.12.14]. Available from http:// clinicalevidence.bmj.com/x/index.html.
15.NimesulidaBoletiminformativodaOMSsobreprodutos farma-cêuticos.2007;5:5-8 [cited16.01.14]. Availablefrom http:// portal.anvisa.gov.br/wps/content/Anvisa+Portal/Anvisa/Pos++ Comercializacao++Pos++Uso/Farmacovigilancia/Assunto+de+ Interesse/Boletins+Informativos.
16.ConsultaRemédios. [cited07.12.14]. Available from http:// consultaremedios.com.br/informacoes-profissionais/busca? termo=nimesulida.
17.Ministério da Saúde. Bulário Eletrônico. [cited 07.12.14]. Available from http://www.anvisa.gov.br/datavisa/filabula/ index.asp.
18.EMEA.EuropeanMedicinesAgencyrecommendsrestricteduse of nimesulide-containing medicinal products. Press release; 2007 [cited 01.02.14]. Available from http://www.ema. europa.eu/docs/enGB/documentlibrary/Pressrelease/2009/ 11/WC500011199.pdf
19.Bulário Eletrônico. Profenid Pediátrico 1mg/mL. [cited 30.11.14]Available from http://www.anvisa.gov.br/datavisa/ filabula/frmResultado.asp#.
20.WiltonLV,PearceG,MannRD.Theuseofnewlymarketeddrugs in children and adolescents prescribed in general practice. In: Special issue: European Society of Pharmacovigilance sixth annualmeeting. 1999 [cited 01.12.14]. Available from http://onlinelibrary.wiley.com/doi/10.1002/(SICI)1099-1557 (199904)8:1+%3CS37::AID-PDS400%3E3.0.CO;2-9/Abstract 21.CellaM,KnibbeC,DanhoFM,DellaPasquaO.Whatistheright
doseforchildren?BrJClinPharmacol.2010;70:597---603. 22.PaulaCS,RapkiewiczJC,SouzaMN,MiguelMD,MiguelOG.
Cen-trodeinformac¸õessobremedicamentoseusoofflabel.RevBras Farm.2010;91:3---8.
23.PereiraGL,DagostiniJM,DalPizzolTS.Alternatingantipyretics inthetreatmentoffeverinchildren:asystematicreviewof randomizedclinicaltrials.JPediatr(RioJ).2012;88:289---96. 24.Nabulsi M. Is combining or alternating antipyretic therapy
morebeneficial thanmonotherapyfor febrilechildren? BMJ. 2009;339:b3540.
25.MelamudA,SuwezdaA,MatamorosR,RingueletL.Indicación deantitérmicospormédicospediatras.Internetcomo modal-idadderecoleccióndedatos.ArchArgentPediatr. 2008;106: 404---8.
26.NationalInstituteforHealthandClinicalExcellence.Feverish illness in children: assessment and initial management in childrenyoungerthan5years;2007[cited28.03.15].Available from http://www.nice.org.uk/guidance/cg160/chapter/1-recommendations
27.Ferreira LA, Ibiapina CC, Machado MGP, Fagundes EDT. Aaltaprevalência deprescric¸õesdemedicamentosoff-label enãolicenciados emunidadedeterapiaintensivapediátrica Brasileira.RevAssocMedBras.2012;58:82---7.
28.AlvesJG,CardosoNetoFJ,AlmeidaCD,AlmeidaND.Dipyrone andacetaminophen:correctdosingbyparents.SaoPauloMed J.2007;125:57---9.
29.BartelinkIH,RademakerCMA,SchobbenAFAM,VanDenAnker JN.Guidelineson paediatricdosingonthebasisof develop-mental physiology and pharmacokinetic considerations. Clin Pharmacokinet.2006;45:1077---97.