w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
Autoimmune
diseases
and
autoantibodies
in
pediatric
patients
and
their
first-degree
relatives
with
immunoglobulin
A
deficiency
Kristine
Fahl
a,
Clovis
A.
Silva
b,c,
Antonio
C.
Pastorino
a,
Magda
Carneiro-Sampaio
a,
Cristina
M.A.
Jacob
a,∗aPediatricAllergyandImmunologyUnit,PediatricDepartment,MedicineSchool,UniversidadeSãoPaulo,SãoPaulo,SP,Brazil
bPediatricRheumatologyUnit,PediatricDepartment,MedicineSchool,UniversidadeSãoPaulo,SãoPaulo,SP,Brazil
cDivisionofRheumatology,MedicineSchool,UniversidadeSãoPaulo,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received23January2014
Accepted6October2014
Availableonline4January2015
Keywords:
IgAdeficiency
Autoimmunity Autoantibodies Thyroiditis
a
b
s
t
r
a
c
t
Introduction:ClinicalmanifestationsofImmunoglobulinADeficiency(IgAD)include
recur-rent infections, atopy and autoimmune diseases. However, to our knowledge, the
concomitantevaluationsofautoimmunediseasesandautoantibodiesinacohortofIgAD
patientswithcurrentage>10yearsandtheirrelativeshavenotbeenassessed.
Objectives: ToevaluateautoimmunediseasesandthepresenceofautoantibodiesinIgAD
patientsandtheirfirst-degreerelatives.
Methods:A cross-sectionalstudy was performed in 34 IgAD patients (current age >10
years) andtheir first-degreerelatives.Allof themwerefollowedat atertiaryBrazilian
primaryimmunodeficiencycenter:27children/adolescentsand7oftheirfirst-degree
rela-tiveswithalatediagnosisofIgAD.Autoimmunediseasesandautoantibodies(antinuclear
antibodies,rheumatoidfactor,andanti-thyroglobulin,anti-thyroperoxidaseandIgAclass
anti-endomysialantibodies)werealsoassessed.
Results:Autoimmunediseases(n=14)and/orautoantibodies(n=10,fourofthemwith
iso-latedautoantibodies)wereobservedin18/34(53%)ofthepatientsandtheirrelatives.The
mostcommonautoimmunediseasesfoundwerethyroiditis(18%),chronicarthritis(12%)
and celiacdisease(6%).The mostfrequent autoantibodieswereantinuclearantibodies
(2%),anti-thyroglobulinand/oranti-thyroperoxidase(24%).Nosignificantdifferenceswere
observedinthefemalegender,ageatdiagnosisandcurrentageinIgADpatientswithand
withoutautoimmunediseasesand/orpresenceofautoantibodies(p>0.05).The
frequen-ciesofprimaryimmunodeficienciesinfamily,autoimmunityinfamily,atopyandrecurrent
infectionsweresimilarinbothgroups(p>0.05).
Conclusion: AutoimmunediseasesandautoantibodieswereobservedinIgADpatients
dur-ing follow-up,reinforcing the necessityof a rigorousand continuousfollow-up during
adolescenceandadulthood.
©2014ElsevierEditoraLtda.Allrightsreserved.
∗ Correspondingauthor.
E-mail:miuki55@uol.com.br(C.M.A.Jacob).
http://dx.doi.org/10.1016/j.rbre.2014.10.003
Doenc¸as
autoimunes
e
autoanticorpos
em
pacientes
pediátricos
e
seus
parentes
de
primeiro
grau
com
deficiência
de
imunoglobulina
Palavras-chave:
DeficiênciadeIgA
Autoimunidade Autoanticorpos Tireoidite
r
e
s
u
m
o
Introduc¸ão: Asmanifestac¸õesclínicasdadeficiênciadeimunoglobulinaA(DIgA)incluem
infecc¸ões recorrentes,atopiae doenc¸asautoimunes.Noentanto,paraonosso
conhec-imento,asavaliac¸õesconcomitantesdedoenc¸asautoimuneseautoanticorposemuma
coortedepacientescomDIgAcomidadeatual>10anoseseusparentesnãoforamfeitas.
Objetivos: Avaliardoenc¸asautoimunesepresenc¸adeautoanticorposempacientescom
DIgAeseusparentesdeprimeirograu.
Métodos: Estudotransversal feitoem 34pacientescomDIgA(idadeatual>10 anos)e
emseusparentesdeprimeirograu.Todosforamacompanhadosemumcentroterciário
brasileiroparaimunodeficiênciaprimária:27crianc¸as/adolescentesesetedeseusparentes
deprimeirograucomdiagnósticotardiodeDIgA.Doenc¸asautoimuneseautoanticorpos
(anticorposantinucleares,fatorreumatoideeantitireoglobulina,antitiroperoxidasee
anti-corposantiendomísiodaclasseIgA)tambémforamavaliadas.
Resultados: Doenc¸asautoimunes (n=14) e/ou autoanticorpos(n=10,quatro delescom
autoanticorposisolados)foramobservadasem18/34(53%)dospacienteseseusparentes.As
doenc¸asautoimunesmaiscomunsencontradasforamtireoidite(18%),artritecrônica(12%)
edoenc¸acelíaca(6%).Osautoanticorposmaisfrequentesforamanticorposantinucleares
(2%),antitireoglobulinae/ouantitireoperoxidase(24%).Nenhumadiferenc¸asignificativafoi
observadanosexofeminino,idadenomomentododiagnósticoeidadeatualempacientes
comDIgAcomesemdoenc¸asautoimunese/oupresenc¸adeautoanticorpos(p>0,05).As
frequênciasdeimunodeficiênciadeprimáriasnafamília,autoimunidadeemfamília,atopia
einfecc¸õesrecorrentesforamsemelhantesemambososgrupos(p>0,05).
Conclusão:Doenc¸asautoimuneseautoanticorposforamobservadasempacientescomDIgA
duranteoacompanhamento,oquereforc¸aanecessidadedeumacompanhamentorigoroso
econtínuoduranteaadolescênciaeaidadeadulta.
©2014ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
IgAdeficiency(IgAD)isthemostfrequentprimary
immuno-deficiency(PID).Itisadefectwhichiscausedduetoterminal
Blymphocytedifferentiation,resultinginaninsufficient
pro-ductionofserumandsecretoryIgA(SIgA).1–4SIgAhassome
protectivefunctionsonmucosa,neutralizingmicroorganisms
andproteins.5–8
TheclinicalmanifestationsofIgADpatients rangefrom
asymptomatictorecurrentinfections,allergicsymptomsand
autoimmune diseases,9 with an increased autoantibodies
production.10Ofnote,autoimmunediseasesoccurin7–36%
ofIgAD patientsand autoantibodiesare observedin more
than40%ofthepatients.10,11TheprevalenceofIgADis1–4%
in systemic lupus erythematosus (SLE) patients,10 2–4% in
rheumatoidarthritis(RA)10and2.6%inceliacdisease(CD).12
Furthermore,autoimmunediseasesarefrequentlyreportedin
relativesofIgADpatients.Amongthefirst-degreerelativesof
IgADpatients,10%hadautoimmunediseasescomparedto5%
ingeneralpopulation.11–15
However,toour knowledge,the concomitant evaluation
ofautoimmunediseasesand autoantibodiesinacohortof
IgADpatientswithcurrentagegreaterthan10yearsandtheir
relativeswithIgADhasnotbeenstudied.
Therefore,theaimofthisstudywastoevaluatethe
occur-renceofautoimmunediseasesandautoantibodiesinacohort
ofIgADpatientswithcurrentagegreaterthan10yearsand
their respective first-degree relatives followed at a tertiary
BrazilianreferencecenterforpediatricPID.
Patients
and
methods
Weselected126IgADpatientsfollowed ataBrazilian
pedi-atricreferencecenterforPIDinthelast30consecutiveyears.
Ninety-twooftheIgADpatientswhowerediagnosedat
child-hoodhadcurrentagelowerthan10yearsandwereexcluded
fromthisstudy.IgAassessmentwassystematicallyperformed
inallfirst-degreerelativesthatpresentedanysymptomsor
signalsofrecurrentinfectiousandautoimmunediseases,and
IgADdiagnosiswasestablishedin7/62(11%)offirst-degree
relatives.Therefore,across-sectionalstudywascarriedoutin
34IgADpatients:27IgADpatients(currentagegreaterthan10
years)andtheir7first-degreerelativeswithalatediagnosisof
IgAD.ThestudywasapprovedbytheEthicalCommitteeand
thewritteninformedconsentwasobtainedfromall
partici-pants.
A systematic clinical evaluation was performed and
included the assessments of various autoimmune
disor-ders, recurrent infectious episodes, atopic manifestations
and current or past neoplasia in the IgAD patients and
their respective families. IgADwas diagnosedaccording to
Table1–Demographicdataandclinicalfeaturesin34IgAdeficiency(IgAD)patientsaccordingtothepresenceof autoimmunediseasesand/orauto-antibodies.
Variables Autoimmunediseasesand/orautoantibodies p
With(n=18) Without(n=16)
Demographicdata
Female 10(56) 9(56) 1.0
AgeatIgADdiagnosis,yrs 8.9(4–34) 13.7(4–52) 0.25
Currentage,yrs 19.8(10–34) 19.6(10–52) 0.98
Clinicalfeatures
PIDinfamily 9(50) 8(50) 1.0
Autoimmunediseasesinfamily 2(11) 3(19) 0.65
Atopy 11(61) 11(69) 0.73
Recurrentinfections 15(83) 16(100) 0.23
Resultsarepresentedinn(%)ormedian(range);PID,primaryimmunodeficiency.
Society forImmunodeficiency with exclusion ofsecondary IgAD.16
Juvenile idiopathic arthritis (JIA) was diagnosed
accord-ingtoInternationalLeagueofAssociationsforRheumatology
(ILAR)criteria.17 Childhood-onsetSLE(c-SLE)wasdiagnosed
according to American Collegeof Rheumatology criteria.18
Ankylosingspondylitis(AS)wasdefinedaccordingtoNewYork
criteria.19 Hypothyroidismwasdefinedasreducedfree
thy-roxine(T4)andelevatedthyroidstimulatinghormone(TSH)
levels.Subclinicalhypothyroidismwasdiagnosedaselevated
TSH associatedwith normal T4. The presence of
antithy-roid antibodies was required to characterize autoimmune
thyroiditis.20 CD was characterized by at least four of the
followingfindings:clinicalmanifestations(chronicdiarrhea,
stuntingand/orirondeficiencyanemia),positivityforCDIgA
antibodies,HLA-DQ2orDQ8genotype,smallintestinebiopsy
compatiblewithceliacenteropathyandresponseto
gluten-freediet.21
Blood samples were collected from all IgAD patients
and their relatives. Serum immunoglobulins (IgA,IgM and
IgG)weremeasured bynephelometry(Behring Laser
Neph-elometer,USA)andtheresultswereconfirmediftheywere
positive.All patientsampleswere screenedforantinuclear
antibodies(ANA)byindirectimmunofluorescencewith
HEp-2 cells (Euroimmun AG, Germany). Dilutions ≥1:80 were
definedaspositive.Rheumatoidfactor(RF)wasdetectedby
immunonephelometry(Laborclin,Pinhais,Parana,Brazil,and
referencevalue>8IU/mL).TheserumlevelsofTSH,free
thy-roxine(freeT4),thyroglobulin,anti-thyroglobulinantibodies
(anti-TG)andanti-thyroperoxidaseantibodies(anti-TPO)were
alsodetermined.TSHandfreeT4weremeasuredby
immuno-metricassays(AutoDelphia,WallacOy,Finland).Theanti-TG
andanti-TPOconcentrationsweredeterminedusing
fluores-cence enzymatic immunoassays (Auto Delphia, Wallac Oy,
Finland;referencevalue>35IU/mL).IgAclassanti-endomysial
(EMA) antibody of IgA isotype was evaluated by indirect
immunofluorescence,using theumbilical cordassubstrate
(Dako,Copenhagen,Denmark;referencevalue>1:10).
Statisticalanalysis
Results were presented as mean±standard deviation or
median(range)forcontinuousandnumber(%)forcategorical
variables.Datawerecomparedbyt-testorMann–Whitneytest
forcontinuousvariables.Differencesofcategoricalvariables
were assessedbyFisher’sexact test.Inallofthestatistical
tests,thelevelofsignificancewassetat5%(p<0.05).
Results
Autoimmune diseases (n=14) and/orautoantibodies (n=10;
fourofthemwithisolatedautoantibodies)wereobservedin
18/34(53%)ofpatientsandtheirrelatives.
The most common autoimmune disorder was thyroid
autoimmune disease in 6 (18%) of the IgAD subjects:
hypothyroidism(n=2),subclinicalhypothyroidism(n=2)and
hyperthyroidism (n=2).Four patientshad chronic arthritis:
threepatientshadJIA(persistentoligoarticularsubtype)and
the fourth patient had AS during adulthood. One patient
hadisolatedhaemolyticautoimmuneanemiaandtheother
patienthadc-SLEwithautoimmunethrombocytopenia.One
patient and his sister had CD with an improvement after
gluten-freediet.
Table1includesdemographicdataandclinicalfeaturesin
34IgADpatientsandtheirfirst-degreerelativesaccordingto
thepresenceofautoimmunediseasesand/orautoantibodies.
Nosignificantdifferenceswereobservedinthefemale
gen-der,ageatdiagnosis andcurrentageinIgADpatientswith
and without the presence ofautoimmune diseases and/or
ofautoantibodies(p>0.05).Likewise,thefrequenciesofPID
and the presenceofautoimmunediseases infamily,atopy
andrecurrentinfectionsweresimilarinbothgroups(p>0.05)
(Table 1).Upper respiratory tract infections were the most
commonfindings,especiallysinusitis(68%)andacutemedia
otitis(58%).
Autoantibodies were observedin10/34(29%)ofpatients
and their first-degreerelatives(four ofthem had
autoanti-bodieswithoutautoimmune diseases).Highserum titersof
anti-TGandanti-TPOantibodieswereobservedinsevenand
fivepatients, respectively.Onefirst-degreerelativewithCD
hadanANAtiterof1:160andonepatientwithc-SLEhadan
ANA titerof1:320. Inthe patientswithoutclinical
autoim-mune manifestations, two ofthem had positivity forboth
anti-TGandanti-TPOantibodiesandtheothertwopatients
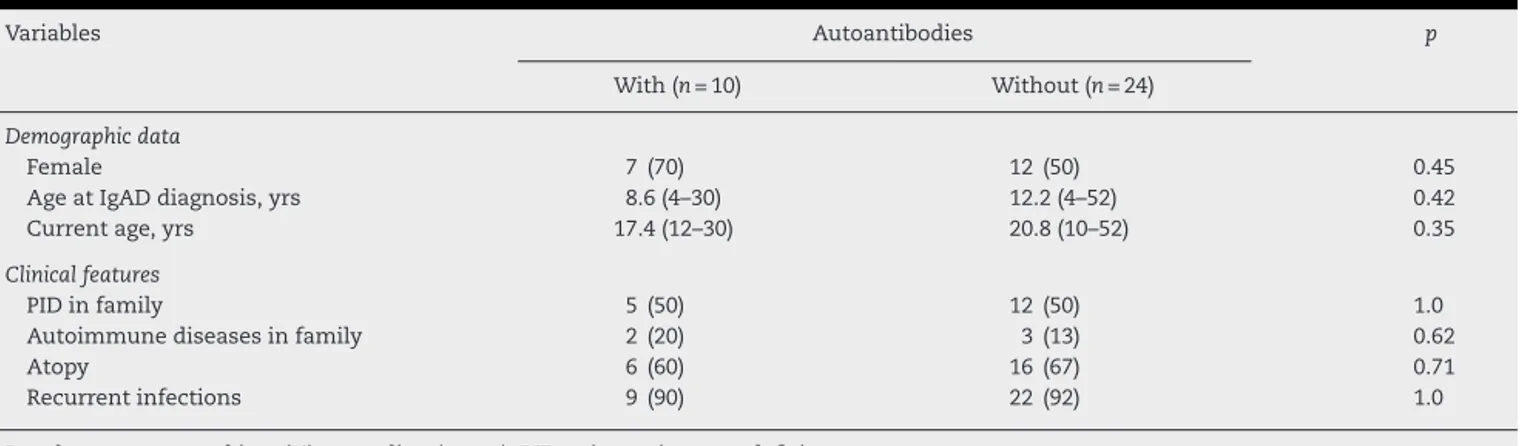
Table2–Demographicdataandclinicalfeaturesin34IgAdeficiency(IgAD)patientsaccordingtopresenceof autoantibodies.
Variables Autoantibodies p
With(n=10) Without(n=24)
Demographicdata
Female 7(70) 12(50) 0.45
AgeatIgADdiagnosis,yrs 8.6(4–30) 12.2(4–52) 0.42
Currentage,yrs 17.4(12–30) 20.8(10–52) 0.35
Clinicalfeatures
PIDinfamily 5(50) 12(50) 1.0
Autoimmunediseasesinfamily 2(20) 3(13) 0.62
Atopy 6(60) 16(67) 0.71
Recurrentinfections 9(90) 22(92) 1.0
Resultsarepresentedinn(%)ormedian(range);PID,primaryimmunodeficiency.
observedinANA-HEp-2cellsofallpatients.RFwasabsencein allIgADsubjects.
Demographicdataandclinicalfeaturesin34IgADpatients accordingto thepresenceofauto antibodies are shown in
Table2.Femalegender,ageatdiagnosisandcurrentagewere
similarinIgADpatientswithandwithoutthepresenceofauto
antibodies(p>0.05).PIDandautoimmunediseasesinfamily,
atopyandrecurrentinfectionswerealsocomparableinboth
groups(p>0.05,Table2).
Discussion
AntibodydeficienciesarethemostcommonPIDinour
Univer-sityHospital,particularlyIgAD.22,23Thepresentstudyshowed
ahighprevalenceofautoimmunediseasesandautoantibodies
inIgADpatientsfollowed-upatatertiaryteachingcenter.
Ofnote,variousclinicalmanifestationsmayoccurinIgAD
patients,rangingfromasymptomatictorecurrentinfections,
allergic symptoms and autoimmune diseases. The clinical
spectrum probably depends on the association with
anti-bodydeficiencies, suchasIgG2subclassdeficiency, specific
antibody deficiency, mannose-binding lectin deficiency or
commonvariableimmunodeficiency.24Otherpossible
patho-genesisisacompensatorymechanism,suchashighsalivary
IgMandIgG.25Inthepresentstudy,noneoftheIgADpatients
hadanyotherPID.
Autoimmune diseases occur more frequently in IgAD
patientscomparedtohealthypopulations,12,22withthe
pos-sibilityofautoantibodiesproduction,evenwithout
autoim-muneclinical manifestations,10,13 asobservedherein. Both
systemicandorgan-specificautoimmunediseaseshavebeen
describedinassociationwithIgAD13 andthemain
autoim-munedisordersassociatedwiththisimmunodeficiencywere:
hypothyroidism,CDandrheumaticdiseases.9–13,26Recently,
IgADhasalsobeenevidencedin4%ofourc-SLEpopulation.27
Additionally,hematologicautoimmunedisordersarevery
frequentinPIDpatients,especiallyantibodydeficiencies,such
as common variable immunodeficiency and IgAD,
particu-larlyidiopathicthrombocytopenicpurpuraandautoimmune
haemolytic anemia, as evidenced in two of our IgAD
patients.28
Importantly,thediagnosisofCDinIgADpatientsmaybe
verydifficult,sincethemajorityofthetestsarebasedon
spe-cificIgAantibody.Therefore,itisimportanttomeasurethe
totalserumIgAlevelsbeforetheCDdiagnosis.29
RegardingpathogenesisofautoimmunityinIgADpatients,
theabsence ofIgAonmucosal surfacesinducesabsorption
ofmanyenvironmentalantigens,suchasdietproteins,and
mayprovokecross-reactionwithself-antigens.30,31Moreover,
the inabilityofdefectiveimmuneresponsetohandlethese
antigensmayresultinacompensatoryresponse,thusleading
totissuedamageandautoimmunity.32,33Inaddition,specific
haplotypes(HLA-A1,HLA-B8andHLA-DR3)werealsofound
inIgADpatientsassociatedwithautoimmunediseases.33
Isolated ANA was also observed in our asymptomatic
patients. Indeed, a Brazilian study showed that 12.6% of
healthychildrenandadolescentsinSãoPaulohadapositive
ANAtiter>1/80,withoutthepresenceofotherautoantibodies.
However,inthisstudyIgAlevelswerenotassessed.34
Auto antibodies were observed in 29% of our IgAD
patients. Thisfrequencywashigherthanourpediatric
lep-rosypatients(16%)35andwaslowerthanourc-SLE,juvenile
dermatomyositis36 and RASopathies37 patients, who
pre-sentedupto93%,59%and52%ofautoantibodies,respectively.
Another relevant result of the present study was the
identificationofIgADinfirst-degreerelatives,mostofthem
asymptomatic,reinforcingtheimportanceofPIDevaluation
in family members. Although many relatives had
autoim-munediseasesandmildinfections,thesemanifestationswere
neglectedbythemandbyphysicians.Therefore,autoimmune
diseasesandinfectiousmanifestationsshouldbeconsidered
intherelativeswithIgAD.
Thisstudy haslimitations.Thesepatients werereferred
to a tertiary university hospital, some ofthem with more
complexdiseases,whichmayhaveoverestimatedthe
autoim-mune disorders in this group. Additionally, infants and
childrenlowerthan10years,whichisthemostprevalentage
groupforthiskindofPID,wereexcluded.Ahealthycontrol
groupwasalsonotassessed.
Inconclusion,ahighprevalenceofautoimmunediseases
andautoantibodieswasobservedinIgADpatients,reinforcing
the rigorous and continuous follow-up duringadolescence
Fundings
ThisstudywassupportedbyFundac¸ãodeAmparoàPesquisa
doEstadodeSãoPaulo(FAPESP–grants2008/58238-4toCAS,
MCSandCMAJ),byConselhoNacionaldoDesenvolvimento
CientíficoeTecnológico(CNPQ–grant302724/2011-7toCAS
and308105/2012-5toCMAJ),byFedericoFoundationtoCAS
andbyNúcleodeApoioàPesquisa“SaúdedaCrianc¸aedo
Adolescente”daUSP(NAP-CriAd).
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. Carneiro-SampaioM,LiphausBL,JesusAA,SilvaCA,Oliveira JB,KissMH.Understandingsystemiclupuserythematosus physiopathologyinthelightofprimaryimmunodeficiencies. JClinImmunol.2008;28:34–41.
2. Carneiro-SampaioMM,CarbonareSB,RozentraubRB,De AraujoMN,RibeiroMA,PortoMH.FrequencyofselectiveIgA deficiencyamongBrazilianblooddonorsandhealthy pregnantwomen.AllergolImmunopathol(Madr). 1989;17:213–6.
3. LeivaLE,ZelazcoM,OleastroM,Carneiro-SampaioM, Condino-NetoA,Costa-CarvalhoBT,etal.Primary immunodeficiencydiseasesinLatinAmerica:thesecond reportoftheLagidregistry.JClinImmunol.2007;27:101–8.
4. KralovicovaJ,HammarströmL,PlebaniA,WebsterAD, VorechovskyI.Fine-scalemappingatIGAD1and
genome-widegeneticlinkageanalysisimplicateHLA-DQ/DR asamajorsusceptibilitylocusinselectiveIgAdeficiencyand commonvariableimmunodeficiency.JImmunol.
2003;170:2765–75.
5. TomasiTB.ThediscoveryofsecretoryIgAandthemucosal immunesystem.ImmunolToday.1992;13:416–8.
6. MesteckyJ,McGheeJR,ImmunoglobulinA.(IgA):Molecular andcellularinteractionsinvolvedinIgAbiosynthesisand immuneresponse.AdvImmunol.1987;40:153–245.
7. LammME.CurrentconceptsinmucosalimmunityIV.How epithelialtransportofIgAantibodiesrelatestohostdefense. AmJPhysiol.1998;274:614–27.
8. SteuerA,McCreaDJ,ColacoCB.PrimarySjogren’ssyndrome, ulcerativecolitisandselectiveIgAdeficiency.PostgradMedJ. 1996;72:499–500.
9. JanziM,KullI,SjöbergR,WanJ,MelénE,BayatN,etal. SelectiveIgAdeficiencyinearlylife:associationtoinfections andallergicdiseasesduringchildhood.ClinImmunol. 2009;133:78–85.
10.LiblauRS,BachJF.SelectiveIgAdeficiencyandautoimmunity. IntArchAllergyImmunol.1992;99:16–27.
11.FusaroAE,FahlK,CardosoEC,deBritoCA,JacobCM, Carneiro-SampaioM,etal.Profileofautoantibodiesagainst phosphorylcholineandcross-reactivitytooxidation-specific neoantigensinselectiveIgAdeficiencywithorwithout autoimmunediseases.JClinImmunol.2010;30:872–80.
12.CataldoF,MarinoV,VenturaA,BottaroG,CorazzaGR. Prevalenceandclinicalfeaturesofselectiveimmunoglobulin Adeficiencyincoeliacdisease:anItalianmulticentrestudy ItalianSocietyofPaediatricGastroenterologyandHepatology (Sigep)andClubdelTenueWorkingGroupsonCoeliac Disease.Gut.1998;42:362–5.
13.ArkwrightPD,AbinunM,CantAJ.Autoimmunityinhuman primaryimmunodeficiencydiseases.Blood.2002;99: 2694–702.
14.Carneiro-SampaioMM,CoutinhoA.Toleranceand autoimmunity:lessonsatthebedsideofprimary immunodeficiencies.AdvImmunol.2007;95:51–82.
15.LemanYel.SelectiveIgAdeficiency.JClinImmunol. 2010;30:10–6.
16.ConleyME,NotarangeloLD,EtzioniA.Diagnosticcriteriafor primaryimmunodeficienciesRepresentingPagid
(Pan-AmericanGroupforImmunodeficiency)andEsid (EuropeanSocietyforImmunodeficiencies).ClinImmunol. 1999;93:190–7.
17.PettyRE,SouthwoodTR,MannersP,BaumJ,GlassDN, GoldenbergJ,etal.InternationalLeagueofAssociationsfor Rheumatologyclassificationofjuvenileidiopathicarthritis: secondrevision,Edmonton,2001.JRheumatol.2004;31:390–2.
18.HochbergMC.UpdatingtheAmericanCollegeof Rheumatologyrevisedcriteriafortheclassificationof systemiclupuserythematosus.ArthritisRheum. 1997;40:1725.
19.VanDerLindenS,ValkenburgHA,CatsA.Evaluationof diagnosticcriteriaforankylosingspondylitis:aproposalfor modificationoftheNewYorkcriteria.ArthritisRheum. 1984;27:361–8.
20.FranklynJA.Hypothyroidism.Medicine(Baltimore). 2005;33:27–9.
21.CatassiC,FasanoA.Celiacdiseasediagnosis:simplerulesare betterthancomplicatedalgorithms.AmJMed.
2010;123:691–3.
22.JacobCM,PastorinoAC,FahlK,Carneiro-SampaioM, MonteiroRC.AutoimmunityinIgAdeficiency:revisitingthe roleofIgAasasilenthousekeeper.JClinImmunol. 2008;28:56–61.
23.Carneiro-SampaioM,Moraes-VasconcelosD,KokronCM, JacobCM,Toledo-BarrosM,DornaMB,etal.Primary immunodeficiencydiseasesindifferentagegroups:areport on1,008casesfromasingleBrazilianreferencecenter.JClin Immunol.2013;33:716–24.
24.SantaellaMR,PeredoR,DisdierOM.IgAdeficiency:clinical correlateswithIgGsubclassandmannan-bindinglectin deficiencies.PRHealthSciJ.2005;24:107–10.
25.BrandtzaegP,KarlssonG,HanssonG,PetrusonB,Björkander J,HansonLA.TheclinicalconditionofIgA-deficientpatients isrelatedtotheproportionofIgD-andIgM-producingcellsin theirnasalmucosa.ClinExpImmunol.1987;67:626–36.
26.EtzioniA.Immunodeficiencyandautoimmunity.Autoimmun Rev.2003;2:364–9.
27.JesusAA,LiphausBL,SilvaCA,BandoSY,AndradeLE, CoutinhoA,etal.Complementandantibodyprimary immunodeficiencyinjuvenilesystemiclupuserythematosus patients.Lupus.2011;20:1275–84.
28.Cunningham-RundlesC.Hematologiccomplicationsof primaryimmunedeficiencies.BloodRev.2002;16:61–4.
29.SinclairD,SaasM,TurkA,GobleM,KerrD.Doweneedto measuretotalserumIgAtoexcludeIgAdeficiencyincoeliac disease?JClinPathol.2006;59:736–9.
30.FairweatherD,KayaZ,ShellanGR,LawsonCM,RoseNR. Frominfectiontoautoimmunity.JAutoimmun.2001;16:341–5.
31.MonteiroRC,VanDeWinkelJG.IgAFcreceptors.AnnuRev Immunol.2003;21:177–204.
32.DeLaatPC,WeemaesCM,GoneraR,VanMunsterPJ, BakkerenJA,StoelingaGB.Clinicalmanifestationsin selectiveIgAdeficiencyinchildhood.Afollow-upreport.Acta PaediatrScand.1991;80:798–804.
34.HilárioMO,LenCA,RojaSC,TerreriMT,AlmeidaG,Andrade LE.Frequencyofantinuclearantibodiesinhealthychildren andadolescents.ClinPediatr(Phila).2004;43:637–42.
35.NederL,RondonDA,CurySS,daSilvaCA.Musculoskeletal manifestationsandautoantibodiesinchildrenand adolescentswithleprosy.JPediatr(RioJ).2014;90:457–63.
36.AikawaNE,JesusAA,LiphausBL,SilvaCA,Carneiro-Sampaio M,VianaVS,etal.Organ-specificautoantibodiesand
autoimmunediseasesinjuvenilesystemiclupus
erythematosusandjuveniledermatomyositispatients.Clin ExpRheumatol.2012;30:126–31.