w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Review
article
Osteoporosis
in
rheumatoid
arthritis:
role
of
the
vitamin
D/parathyroid
hormone
system
Mattia
Bellan
a,∗,
Mario
Pirisi
a,b,
Pier
Paolo
Sainaghi
a,baInternalMedicineandRheumatologyUnit,AOUMaggioredellaCarità,Novara,Italy
bInterdisciplinaryResearchCenteronAutoimmuneDiseases(IRCAD),DepartmentofTranslationalMedicine,UniversitàdelPiemonte
OrientaleA.Avogadro,Novara,Italy
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received25February2014 Accepted6October2014 Availableonline6January2015
Keywords:
Cholecalciferol Parathyroidhormone Osteoporosis Rheumatoidarthritis
a
b
s
t
r
a
c
t
Osteoporosisisawell-establishedextra-articularfeatureofrheumatoidarthritis(RA). Sys-temic inflammationseems to play a crucial role in causing an alteration ofmultiple homeostaticsystemsimpliedinbonehealth,suchastheRANK/RANKL/Osteoprotegerinand Wnt/cateninpathways;severalothercausalfactorshavebeencalledintoquestion, includ-ingthechronicuseofcorticosteroids.SincevitaminDexertsimportantimmune-regulatory roles,ithasbeenclaimedthatderangementofthevitaminD/parathyroidhormone(PTH) system,awell-knowndeterminantofbonehealth,mayplayapathogenicrolein autoim-munity;animalmodelsandclinicaldatasupportthishypothesis.Furthermore,RApatients seemtoberelativelyrefractorytovitaminD-inducedPTHsuppression.Therefore,thelink betweenRAandosteoporosismightinpartbeduetoalterationsinthevitaminD/PTH sys-tem.Abetterunderstandingofthepathophysiologyofthissystemmaybecrucialtoprevent andcureosteoporosisinpatientswithinflammatory/autoimmunediseases.Amajor clin-icalcorrelateofthestrictcooperationandinterdependencebetweenvitaminDandPTH isthatcorrectionofthevitaminDdeficiency,atleastinautoimmunediseases,shouldbe targetedtoPTHsuppression.
©2014ElsevierEditoraLtda.Allrightsreserved.
Osteoporose
na
artrite
reumatoide:
papel
do
sistema
vitamina
D/hormônio
paratireóideo
Palavraschave:
Colecalciferol
Hormônioparatireóideo Osteoporose
Artritereumatoide
r
e
s
u
m
o
A osteoporose é uma característicaextra-articularbem estabelecida da artrite reuma-toide (AR). A inflamac¸ão sistêmica parece ser essencial para causar uma alterac¸ão em múltiplos sistemas homeostáticos implicados na saúde óssea, como as vias RANK/RANKL/osteoprotegerinaeWnt/catenina;váriosoutrosfatorescausaistêmsido implicados,comoousocrônicodecorticosteroides.ComoavitaminaDexercefunc¸ões
∗ Correspondingauthor.
E-mail:bellanmattia@yahoo.it(M.Bellan).
http://dx.doi.org/10.1016/j.rbre.2014.10.007
imunorreguladorasimportantes,tem-seafirmadoqueodesarranjodosistemavitamina D/hormônioparatireóideo(HPT),umdeterminantebemconhecidodasaúdeóssea,pode desempenharumpapelpatogêniconaautoimunidade;estudoscomanimaisedados clíni-cosapoiamessahipótese.Alémdisso,ospacientescomARparecemserrelativamente refratáriosàsupressãodeHPTinduzidapelavitaminaD.Portanto,aligac¸ãoentreaARe aosteoporosepodeserempartecausadaporalterac¸õesnosistemavitaminaD/HPT.Uma melhorcompreensãodafisiopatologiadessesistemapodesercrucialparaprevenirecurar aosteoporoseempacientescomdoenc¸asinflamatórias/autoimunes.Amaiorevidênciada correlac¸ãoclínicadecooperac¸ão einterdependênciaentrea vitaminaDeoHPTéque acorrec¸ãodadeficiênciadevitaminaD,pelomenosnasdoenc¸asautoimunes,deveser orientadaparaasupressãodoHPT.
©2014ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Osteoporosis is a frequent complication of autoimmune inflammatory diseases, such as rheumatoid arthritis (RA), ankylosing spondylitis(AS), systemic lupus erythematosus (SLE)andmultiplesclerosis(MS).1Thereasonswhy
osteoporo-sisoccursinthesediseasesaremultipleandnotcompletely understood.Thefailureofseveralboneregulationsystemshas beenclaimedtoberesponsibleforthiscomplicationof sys-temicinflammatorydiseaseseventhoughthisissueremain partiallyunresolved.
ThevitaminD/PTHsystemisawellknowndeterminant ofbonehealthinthegeneralpopulation.Recently,afailurein VitaminDmetabolismhasbeendescribedinpatientsaffected byinflammatoryrheumaticdiseases,2 eventhoughits
rela-tionshipwiththepathogenesisofautoimmunediseasesand the consequences on bone health remain not completely understood.Inthispaperwewillreviewtheroleofthevitamin D/PTHsysteminthepathogenesisofosteoporosisin autoim-munediseasesand,inparticular,inRA.
Toperformthe present review,weinterrogatedPubMed (accessedon-line on July 1st, 2013),limiting our search to paperspublishedinEnglishuntilJune30th,2013,andusing the followingstring: “(vitaminD PTH system) OR (vitamin Drheumatoidarthritis)OR(rheumatoidarthritissecondary hyperparathyroidism) OR (rheumatoid arthritis hypovita-minosisD)”yielded856articles.Eight-hundredpaperswere excludedforthefollowingreasons:(a)dealtwithtopics non-relevantforthe present review,(b)letters, casereports, (c) smallsamplesize,(d)unobtainablefulltextarticles,ora com-binationoftheabovereasons.Wereviewedalltheremaining papers,plusadditionalrelevantarticlesidentifiedfrom the referencesofselectedarticlesorthroughpersonalknowledge oftheauthors.
Osteoporosisandautoimmuneinflammatorydiseases
Osteoporosisisaclinicalconditioncharacterizedbyahigh riskofvertebralandnon-vertebralfractures,duetothe reduc-tionofBoneMineralDensity(BMD).Italsorepresentsawell establishedextra-articularfeatureofRA.3
PatientsaffectedbyRAhavebeenreportedtobeathigher riskofvertebralandnonvertebralfractures.4,5 Withrespect
tothereference populationvalues,femaleRApatients dis-play lower bone mineral density (BMD) values at the hip and the spine;the risk ofosteoporosisseemsto behigher amongpatientswhoareolder,postmenopausal,positivefor RheumatoidFactor,treatedwithcorticosteroids,havelonger diseasedurationandhigherburdenofdisability.6 Recently,7
inaperspectivecohortof102RApatientswhocompleteda 5-yearsfollow-up,anannualincidenceofvertebralfractures of3.7/100patients/yearhasbeenreported,higherthaninthe generalpopulationaccordingtootherprospectivestudies.8,9
The annual incidence of non vertebral fractures was also increased.Thesedatahavebeenconfirmedbyotherstudies10
inwhichtheriskofallfracturesinRApatientswas1.5-fold higherthanhealthycontrols.
However,theincreasedriskofosteoporosisisnotlimited toRApatientsbuthasbeenalsoreportedforother autoim-munediseases suchasMS,AS,and SLE.1 Thereasonswhy
patientsaffectedbyautoimmuneinflammatorydiseasesare prone todevelop osteoporosis are complex.A central role seemstobeplayedbysystemicinflammation.Receptor Acti-vatorofNuclearFactorKappaB(RANK)isanuclearreceptor expressedbyosteoclastprecursors and matureosteoclasts, whichmediatesosteoclastogenesisafterbindingtoitsligand, RANKL.11InpatientswithRAtheoverexpressionofseveral
inflammatorycytokines(TNF-␣,interleukin(IL)-1,IL-6and IL-17)favorstheactivation,differentiationandproliferationof osteoclastsinducedbyRANKL.12,13Thissystemisfurther
reg-ulatedbyosteoprotegerin(OPG),adecoyreceptorexpressed byosteoblastswhichcompeteswithRANKforthebindingto RANKL;so,OPGisaninhibitorofosteoclastactivationinvitro andinvivo.14
FurthermoreTNF␣canalsoinduceosteocytestosynthetize sclerostinandDkk-1,15twoinhibitorsoftheWnt/catenin
pathway,acrucialsystemforosteoblasticdifferentiation.16,17
7-de hydrocholesterol
25-hydroxylation
) H O ( 5 2 -4 2 ) Cholecalciferol
H O ( 5
2 2 Cholecalciferol
1-hydroxylation 24-hydroxylation
1-25(OH)2 Cholecalciferol 1-24-25(OH)
3 Cholecalciferol
Cholecalciferol
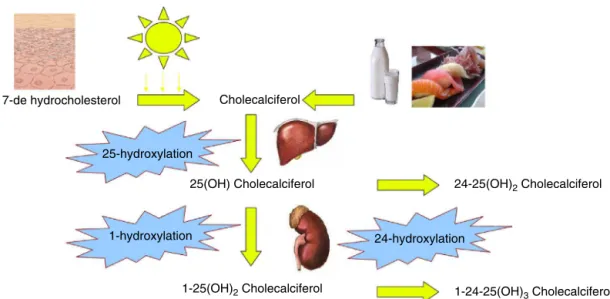
Fig.1–VitaminDmetabolism.Cholecalciferol,bothderivingfromdietaryintakeandfromendogenoussynthesis,circulates inthebloodboundtoavitaminDbindingprotein.Then,itishydroxylatedto25(OH)cholecalciferolbyaliverenzymeand furtherhydroxylatedto1,25(OH)2DbyCYP27B1,expressedbykidney,butalsobyseveralothertissues.Theinactivationis
mediatedby24-hydroxylation.
inflammation;furthermore,sclerostinincreasedwhileDkk-1 decreasedwithrespecttobaseline.18
Similardatahavealsobeenobtainedwithotherbiologics; infact,theimprovementofinflammationcontrolwith inflix-imabhasbeenassociatedwithareductioninboneloss.19
Oneothermajorfactorinthepathogenesisofosteoporosis inrheumaticdiseasesisthelongtermuseofcorticosteroids. It is known that glucocorticoids can induce osteoporosis throughdifferentmechanisms20:infact,theuseof
glucocor-ticoidsreducesthenumberandthefunctionofosteoblasts21
andimpairs theirdifferentiationand maturation22 through
interferencewithWnt/-cateninsignaling.23Inthiscontext,
theapoptosisofosteoblastsandosteocytesisenhanced,24the
expressionofRANK-LincreasedandthatofOPGdecreased25
favoringtheactivationofosteoclasts.Intheclinicalsetting glucocorticoid treatment is an independent risk factor for boneloss26;inameta-analysison2891steroidusers
gluco-corticoidtreatmenthasbeenlinkeddosedependentlytobone lossand riskfractures, inparticularinthe first monthsof treatment.Theriskdecreasesafterstoppingtherapy.However, dosesaslowas5mg/dayhavebeenreportedtoincreasethe riskoffractures ofapproximately20%;interestingly,higher initial doses are stronger related to the risk of bone loss thanhighercumulativedoses.27AccordingtoACRguidelines,
any patient starting a long term (>3 months) steroid reg-imen should receive calcium and vitamin D; furthermore, bisphosphonatesshouldbestartedaccordingtotheassessed osteoporosisrisk.28
VitaminDmetabolism
VitaminDisasecosteroidhormonethatinthebodyisderived bothfromdietaryintakeandendogenoussynthesis.Several foodsaredietarysourcesofVitaminD,eitherasVitaminD2
(ergocalciferol)orVitaminD3(cholecalciferol),includingcod
oil,fishandfortifiedmilk.However,thegreaterpartof Vita-minDrequiredformetabolismoriginatesfromendogenous synthesis. The metabolic pathways start from the activa-tionofaprecursor(7-dehydrocholesterol)intheskinwhich is photolysed into cholecalciferol following sun exposure; cholecalciferolisthenhydroxylatedto25-hydroxyvitaminD (25(OH)D)bydifferentisoformsofa25-hydroxylase(CYP2C11, CYP2J3,CYP2R1,CYP3A2,CYP27A1)intheliver.25(OH)Disthe circulatingformofthevitamin,boundtoVitaminDbinding protein(DBP).25(OH)Dcanalsobestoredinfattissue,andis theintermediatemetaboliteusuallymeasuredtodefinethe vitaminDstatus,becauseitismorestableandhasalonger lifethantheactiveform.29
1,25-DihydroxyvitaminD(1,25(OH)2D,alsocalledcalcitriol)
representsthe activeform,and itisthe resultofafurther hydroxylation step mediatedby 1-␣-hydroxylase (CYP27B1) expressed in kidney (Fig. 1). This is the most important check-pointofVitaminD metabolismandCYP27B1 activity isstrictlycontrolled:inparticularPTHinducesitsactivity,30
while high serum calcium concentration and 1,25(OH)2D
downregulateits expression.31Whiletheexpressionof
25-hydroxylase seemsto berestrictedtothe liver, CYP27B1is expressedbymanyothertissues,includingplacenta, endothe-lium,prostate,monocytesandmacrophages,skin,colonand brain.32
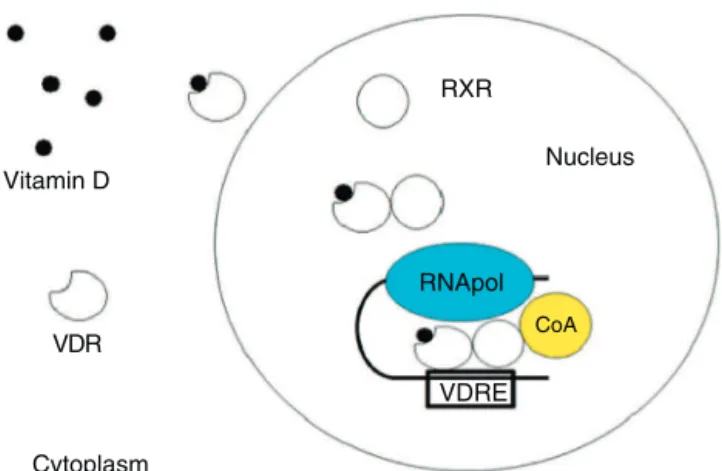
1,25(OH)2D acts on its target cells through a nuclear
receptor (vitaminD receptor – VDR).The bindingbetween 1,25(OH)2DandVDRinducestheheterodimerizationwithRXR
Vitamin D
VDR
RXR
Nucleus
VDRE RNApol
CoA
Cytoplasm
Fig.2–MechanismofactionofvitaminD.Theactiveform ofvitaminDreachesthecytoplasm,whereitbindstoVDR (vitaminDreceptor),inducingnucleartranslocationand furtherassociationtoRXR(retinoidXreceptor),thereby creatingatranscriptionalcomplextogethertoRNA
polymerase(RNApol)andaco-activator(CoA)abletoinduce orrepressgenestranscription.Thiscomplexrecognizes specificDNA-sequences,alsoknownasVDRE(VitaminD responsiveelements).
VitaminD/PTHsystemandbonehealth
TheclassicandbestdefinedfunctionofvitaminDisthe regu-lationofcalcium/phosphorusmetabolism,whichisessential togrant bone health. In particular 1,25(OH)2D inducesgut
absorption of calcium and phosphorus34; furthermore it
actsonkidney tubulesdetermininganincreaseincalcium reabsorption.35Theglobalresultisanincreaseinplasma
cal-ciumandphosphorusconcentration.
1,25(OH)2Dhasvariouseffectsonbonecells:inparticular,
itincreasestheexpressionofosteopontin36andosteocalcin37
inosteoblasts,increasestheRANKLexpressionontheplasma membrane of osteoblasts and inhibits the synthesis of OPG. Thereby, vitamin D increases the number of RANKL moleculesabletobindtoRANK,allowingaphysiologicalbone turnover.38
It is well known that vitamin D actions on bone are strictlylinkedtoPTHactivity,becausevitaminDneedsPTH toplayits role on bone,but alsobecause vitaminD down regulatesPTHsynthesisbothindirectly(increasing calcium concentration) and directly (activating a VDRE in the pro-moterofthePTHgene).VitaminDalsoinhibitsparathyroid cells proliferation39 and modulates the sensitivity to
cal-cium,increasingthetranscriptionofCasR(CalciumSensing Receptor).40
Thesemolecularpathwayshaveimportantclinical impli-cations.First,eventhoughinthelastdecadeotherbonehealth determinantshavebeenusedtodefinevitaminDstatus,the definition of a normal plasma vitamin D concentration is mainlybasedontheidentificationofa25(OH)Dplasma con-centrationabletosuppressPTH.Thisthresholdofnormality hasbeensetbysomeat30ng/mL(75nmoL/L).41
Therefore,patientsaffectedbyseverevitaminDdeficiency cansufferasecondaryhyperparathyroidismresponsibleofan inadequatebonemineralizationleading toricketsor osteo-malacia; inthe modern era these conditions havebecome rare, although hypovitaminosis D isextremely common in the general population. The impact of hypovitaminosis D onboneoutcomesisstilldebated,inparticularwithregard totherelationshipbetweenlowervitaminDlevelsand the riskoffallsand fractures.InasystematicreviewvitaminD concentrationcorrelated positively withBMDand inversely withtheriskoffalls,42whileacorrelationwithanincreased
risk of fractures lacked consistence. These data confirmed the direct relationship between vitamin D concentrations andBMDobservedinalargepopulationofpostmenopausal women,43 as well as in the community-dwelling men and
women aged atleast 20 years who participated to the US NHANES III survey.44 However,recently, hypovitaminosis D
has been also associatedto an increased risk of vertebral fractures.45
Eventhoughthedefinitionofnormal25(OH)Dplasma con-centrationisstilldebated,aclassificationofvitaminDstatus onwhichmanywouldagreeis:deficiency25(OH)D<20ng/mL (<50nmoL/L),insufficiency20–30ng/mL(50–75nmoL/L), nor-mality>30ng/mL(>75nmoL/L).46
VitaminDsupplementationseemstobelinkedto favor-ableclinicaloutcomes.Inparticulartheresolutionofvitamin D deficiencyis associatedwitharecovery inbonemineral density47; furthermore vitaminD supplementationreduces
the riskoffalls and fracturesaccording tothe resultsofa meta-analysis.48,49
Itwassuggestedthata25(OH)Dplasmaconcentrationof at least60nmoL/Lis necessary forprevention offalls and fractures.50 However the preferable regimen of vitamin D
supplementation has notbeen established yet and several differentschemeshavebeensuggested.51–53Recently46
pub-lishedguidelinessuggestedtheuseofhighdoses(2000IU/day or50,000IUonceweeklyfor6weeks)ergocalciferolor chole-calciferolinthecorrectionofhypovitaminosisD,followedby amaintenancedailydoseof400–1000IU/day.
However, a randomized clinical trial in 2010 surpris-ingly reported an increased risk of falls and fractures in patients treated with onesingle annual dose of 500,000IU cholecalciferol.54Althoughthesedataawaitconfirmationin
otherstudies,thisobservationquestionthesafetyof admin-isteringsinglehighdosecholecalciferol.
VitaminDinautoimmunediseases
Inthepastfewyears,severalreportspointedoutaputative rolefortheactivemetabolite1-25(OH)2Dinimmunesystem
regulation.Thishypothesisisbasedontheobservationsthat 1-25(OH)2Dhasimmuno-modulatoryeffects oncells ofthe
innateimmunity.Infact,invitro,itinducesthe differentia-tionofmonocyteswithinhibitionofinflammatorycytokines production(TNF-␣,IL-6,IL-1).Moreover,1-25(OH)2Ddecreases
TheimportanceofvitaminDmetabolitesinimmune regu-lationisconfirmedbyrecentdatashowingthatmacrophages and monocyte-derived DCs express the enzyme CYP27B1. In this way, 1-25(OH)2D is generated locally and binds
to VDR in immune cells, thereby exerting concentration-dependentanti-inflammatoryautocrineandparacrineeffects in lymphoid microenvironments.32 In addition, some
epi-demiological studies have correlated hypovitaminosis D to the development of autoimmune diseases, such as multi-ple sclerosis, type I diabetes, systemic sclerosis, SLE and RA.58 Infact, alowerdietary intakeofvitaminDhasbeen
linked to a higher risk of RA development in a meta-analysis ofseveral studies59; furthermore, plasma 25(OH)D
concentrationhasbeenreportedtobelowerinRApatients whencomparedtohealthycontrols,60althoughtheseresults
werenotconfirmedinotherstudies.61Plasma25(OH)D
con-centration hasalso been inversely correlated with disease activity.59,62
Further evidence for an involvement of vitamin D metabolisminthe pathogenesisofRAisbasedon the fol-lowingobservations,suggestingalocalactionforvitaminD metabolitesinthemodulationofjointinflammation:
1,25(OH)2Dand25(OH)Daredetectableinsynovialfluid63;
VDRisexpressedinthesynovialmembraneofRApatients64;
VDR-knock-outmicedevelopamoreaggressiveformof TNF-inducedarthritiswithrespecttomicewithanormalvitamin Dfunction65;
TNF-blockade requires the presence of 1,25(OH)2D to
suppressTh17activityand,consequently,synovial inflam-mationinjointtissuescultures.66
TheimportanceofunderstandingtheroleofvitaminDin autoimmunediseasesisenforcedbytheveryhighprevalence ofhypovitaminosisDinthisparticularsetting.61,67
RelativehypovitaminosisDandsecondary
hyperparathyroidisminautoimmunerheumaticdiseases
AnimpairmentofVitaminDsystemhasalreadybeen postu-latedasaconcausalfactorinthepathogenesisofosteoporosis ininflammatoryarthritis.Infact, aVDRpolymorphism has beenlinkedtobonelossinRA;inparticular68Rassand
col-leaguesfoundalowerBMDinRApatientscarryingtheBBand BbgenotypesoftheVDRBsmIpolymorphismwithrespectto carriersofthebbgenotype.TheseresultssuggestthattheB allelemaybeamarkerforincreasedbonereabsorptionand bonelossinRA.Inaddition,werecentlydescribeda signif-icantlyhigherPTHconcentrationin105patientsaffectedby autoimmunerheumaticdiseaseswithrespectto1020controls despitesimilarplasmavitaminDconcentration.These find-ingsheldtruealsocategorizingpatientsindifferentgroups accordingtodifferentvitaminDthresholds.Wealsoobserved thatpatients showedahigher prevalenceof hyperparathy-roidismthancontrols.Finally,atstepwiselogisticregression analysis,plasma25(OH)D<75nmoL/L,age≥65yearsandthe
presenceofanautoimmunerheumaticdiseasewere indepen-dentpredictorsforhyperparathyroidism.2
This result has been confirmed in a further paper, in which we observed that suppression of secondary
hyperparathyroidisminautoimmunerheumaticpatientswas impaired withrespecttopatientsaffected byosteoarthritis after a cholecalciferol regimen used to correct hypovita-minosisD.69
Similarly, ina multicentric study,RApatients with ero-siveevolutionpresentedwithhigherPTHandlowerBMDin comparisontopatientswithlessaggressivearthritis,despite possessingsimilarvitaminDconcentrations.70
Theseobservationsraisetheattentiononthepossible pres-ence ofa “relative hypovitaminosis D” inRA and in other inflammatory rheumaticdiseases,which could explainthe reduction in the physiological actions of this molecule; in these patients, indeed, the mechanisms that regulate PTH synthesisseemtobemorerefractorytovitaminD suppres-sion.
Apossibleexplanationforthesefindingsisthatchronic inflammation may reduce parathyroid cells sensitivity to 1,25(OH)2D; the reduced sensitivity of parathyroid cells
could reflect a more complex refractoriness to 1,25(OH)2D
actions, involving immune cells too. Alternatively, since the active vitamin D metabolite has immunosuppressive andimmunoregulatoryeffects,duringchronicinflammation, immunecellsmayconsumehigherquantitiesof1-25(OH)2D,
loweringitsavailabilitytoparathyroidcellswithconsequent PTHhyperproduction.
Sointhissetting,thevitaminD/PTHsystemseemstobe somehowalteredandthisdysfunctioncouldparticipate sig-nificantly inthepathogenesisofosteoporosisinrheumatic patients,andmayrepresentapromisingtherapeutictarget.
Atthebestofourknowledge,veryfewstudiesevaluatedthe bestsupplementationregimenofcholecalciferolinrheumatic patients.Wehavedemonstratedthatonlyahighdose supple-mentation regimen (300,000IU orally once) followed by a maintenancedose was superior,alsoif stillsuboptimal,in thecorrectionofhypovitaminosisDandofsecondary hyper-parathyroidisminrheumaticpatients.70
Conclusions
Inthelastfewyearsthesamedefinitionof“vitaminD”has beendebatedandremainsthesubjectofintensecontroversy. Infact,vitaminDisnotonlyobtainedbydietaryintake,likethe othervitamins,sinceitsgreateramountderivesfrom endoge-noussynthesis.Importantly,theconceptof“vitamin”isstatic, whilevitaminDandPTHparticipateindynamicsystem act-ingonseveralbiologicaltargets.ForthesereasonsvitaminD iscurrently,morecorrectly,definedas“hormoneD”;infact the relationship betweenvitamin D and PTH shares many similaritieswiththeotherendocrinenetworks.
aremeasuredtoestablishtheneedforcholecalciferol supple-mentation,andthatVitaminDsupplementationistargeted tothecorrectionofhyperparathyroidismratherthan tothe normalizationofplasma25(OH)Dconcentrationalone.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. BultinkIE,VisM,VanderHorst-BruinsmaIE,LemsWF. Inflammatoryrheumaticdisordersandbone.CurrRheumatol Rep.2012;14:224–30.
2. SainaghiPP,BellanM,AntoniniG,BellomoG,PirisiM. Unsuppressedparathyroidhormoneinpatientswith autoimmune/inflammatoryrheumaticdiseases:implications forvitaminDsupplementation.Rheumatology.
2011;50:2290–6.
3. DeodharAA,WoolfAD.Bonemassmeasurementandbone metabolisminrheumatoidarthritis:areview.BrJRheumatol. 1996;35:309–22.
4. PeelNF,MooreDJ,BarringtonNA,BaxDE,EastellR.Riskof vertebralfractureandrelationshiptobonemineraldensityin steroidtreatedrheumatoidarthritis.AnnRheumDis. 1995;54:801–6.
5. KimSY,SchneeweissS,LiuJ,DanielGW,ChangCL,Garneau K,etal.Riskofosteoporoticfractureinalarge
population-basedcohortofpatientswithrheumatoid arthritis.ArthritisResTher.2010;12:R154.
6. HaugebergG,UhligT,FalchJA,HalseJI,KvienTK.Bone mineraldensityandfrequencyofosteoporosisinfemale patientswithrheumatoidarthritis:resultsfrom394patients intheOsloCountyRheumatoidArthritisregister.Arthritis Rheum.2000;43:522–30.
7. VisM,HaavardsholmEA,BøyesenP,HaugebergG,UhligT, HoffM,etal.Highincidenceofvertebralandnon-vertebral fracturesintheOSTRAcohortstudy:a5-yearfollow-upstudy inpostmenopausalwomenwithrheumatoidarthritis. OsteoporosInt.2011;22:2413–9.
8. EposStudyGroup.IncidenceofvertebralfractureinEurope: resultsfromtheEuropeanProspectiveOsteoporosisStudy (Epos).JBoneMinerRes.2002;17:716–24.
9. NevittMC,CummingsSR,StoneKL,PalermoL,BlackDM, BauerDC,etal.Riskfactorsforafirst-incidentradiographic vertebralfractureinwomenatleast65yearsofage:thestudy ofosteoporoticfractures.JBoneMinerRes.2005;20:131–40.
10.VanStaaTP,GeusensP,BijlsmaJW,LeufkensHG,CooperC. Clinicalassessmentofthelong-termriskoffracturein patientswithrheumatoidarthritis.ArthritisRheum. 2006;54:3104–12.
11.HsuH,LaceyDL,DunstanCR,SolovyevI,ColomberoA, TimmsE,etal.Tumornecrosisfactorreceptorfamilymember RANKmediatesosteoclastdifferentiationandactivation inducedbyosteoprotegerinligand.ProcNatlAcadSciUSA. 1999;96:3540–5.
12.GeusensPP,LemsWF.Osteoimmunologyandosteoporosis. ArthritisResTher.2011;13:242.
13.TakayanagiH.Osteoimmunologyandtheeffectsofthe immunesystemonbone.NatRevRheumatol.2009;5:667–76.
14.KongYY,YoshidaH,SarosiI,TanHL,TimmsE,CapparelliC, etal.OPGLisakeyregulatorofosteoclastogenesis, lymphocytedevelopmentandlymph-nodeorganogenesis. Nature.1999;397:315–23.
15.SchettG,SaagKG,BijlsmaJW.Frombonebiologytoclinical outcome:stateoftheartandfutureperspectives.AnnRheum Dis.2010;69:1415–9.
16.RachnerTD,KhoslaS,HofbauerLC.Osteoporosis:nowand thefuture.Lancet.2011;377:1276–87.
17.LoriesRJ,LuytenFP.Osteoimmunology:Wntantagonists:for betterorworse?NatRevRheumatol.2009;5:420–1.
18.TerposE,FragiadakiK,KonstaM,BratengeierC,
PapatheodorouA,SfikakisPP.EarlyeffectsofIl-6receptor inhibitiononbonehomeostasis.ClinExpRheumatol. 2011;29:921–5.
19.VisM,HavaardsholmEA,HaugebergG,UhligT,VoskuylAE, vandeStadtRJ,etal.Evaluationofbonemineraldensity, bonemetabolism,osteoprotegerinandreceptoractivatorof theNFkappaBligandserumlevelsduringtreatmentwith infliximabinpatientswithrheumatoidarthritis.AnnRheum Dis.2006;65:1495–9.
20.CanalisE,MazziottiG,GiustinaA,BilezikianJP.
Glucocorticoid-inducedosteoporosis:pathophysiologyand therapy.OsteoporosInt.2007;18:1319–28.
21.CanalisE.EffectofglucocorticoidsontypeIcollagen synthesis,alkalinephosphataseactivity,and
deoxyribonucleicacidcontentinculturedratcalvariae. Endocrinology.1983;112:931–9.
22.ItoS,SuzukiN,KatoS,TakahashiT,TakagiM.Glucocorticoids inducethedifferentiationofamesenchymalprogenitorcell line,ROB-C26intoadipocytesandosteoblasts,butfailto induceterminalosteoblastdifferentiation.Bone. 2007;40:84–92.
23.OhnakaK,TanabeM,KawateH,NawataH,TakayanagiR. GlucocorticoidsuppressesthecanonicalWntsignalin culturedhumanosteoblasts.BiochemBiophysResCommun. 2005;329:177–81.
24.O’BrienCA,JiaD,PlotkinLI,BellidoT,PowersCC,StewartSA, etal.Glucocorticoidsactdirectlyonosteoblastsand osteocytestoinducetheirapoptosisandreducebone formationandstrength.Endocrinology.2004;145:1835–41.
25.HofbauerLC,GoriF,RiggsBL,LaceyDL,DunstanCR,
SpelsbergTC,etal.Stimulationofosteoprotegerinligandand inhibitionofosteoprotegerinproductionbyglucocorticoidsin humanosteoblasticlineagecells:potentialparacrine mechanismsofglucocorticoid-inducedosteoporosis. Endocrinology.1999;140:4382–9.
26.DeNijsRN,JacobsJW,BijlsmaJW,LemsWF,LaanRF,Houben HH,etal.Prevalenceofvertebraldeformitiesand
symptomaticvertebralfracturesincorticosteroidtreated patientswithrheumatoidarthritis.Rheumatology. 2001;40:1375–83.
27.VanStaaTP,LeufkensHG,CooperC.Theepidemiologyof corticosteroid-inducedosteoporosis:ameta-analysis. OsteoporosInt.2002;13:777.
28.GrossmanJM,GordonR,RanganathVK,DealC,CaplanL, ChenW,etal.AmericanCollegeofRheumatology2010 recommendationsforthepreventionandtreatmentof glucocorticoid-inducedosteoporosis.ArthritisCareRes. 2010;62:1515.
29.ZerwekhJE.BloodbiomarkersofvitaminDstatus.AmJClin Nutr.2008;87:1087S–91S.
30.GarabedianM,HolickMF,DelucaHF,BoyleIT.Controlof 25-hydroxycholecalciferolmetabolismbyparathyroidglands. ProcNatlAcadSciUSA.1972;69:1673–6.
31.GhazarianJG,JefcoateCR,KnutsonJC,Orme-JohnsonWH, DeLucaHF.Mitochondrialcytochromep450.Acomponentof chickkidney25-hydrocholecalciferol-1alpha-hydroxylase.J BiolChem.1974;249:3026–33.
32.AdamsJS,HewisonM.Extrarenalexpressionofthe
33.MeyerMB,PikeJW.Corepressors(NCoRandSMRT)aswellas coactivatorsarerecruitedtopositivelyregulated
1␣,25-dihydroxyvitaminD(3)-responsivegenes.JSteroid BiochemMolBiol.2013;136:120–4.
34.FleetJC,SchochRD.Molecularmechanismsforregulationof intestinalcalciumabsorptionbyvitaminDandotherfactors. CritRevClinLabSci.2010;47:181–95.
35.JonesG,StrugnellSA,DeLucaHF.Currentunderstandingof themolecularactionsofvitaminD.PhysiolRev.
1998;78:1193–231.
36.NodaM,VogelRL,CraigAM,PrahlJ,DeLucaHF,DenhardtDT. IdentificationofaDNAsequenceresponsibleforbindingof the1,25-dihydroxyvitaminD3receptorand
1,25-dihydroxyvitaminD3enhancementofmousesecreted phosphoprotein1(SPP-1orosteopontin)geneexpression. ProcNatlAcadSciUSA.1990;87:9995–9.
37.BortellR,OwenTA,BidwellJP,GavazzoP,BreenE,vanWijnen AJ,etal.VitaminD-responsiveprotein–DNAinteractionsat multiplepromoterregulatoryelementsthatcontributetothe levelofratosteocalcingeneexpression.ProcNatlAcadSciU SA.1992;89:6119–23.
38.KimS,YamazakiM,ZellaLA,MeyerMB,FretzJA,ShevdeNK, etal.Multipleenhancerregionslocatedatsignificant distancesupstreamofthetranscriptionalstartsitemediate RANKLgeneexpressioninresponseto1,25-dihydroxyvitamin D3.JSteroidBiochemMolBiol.2007;103:430–4.
39.CozzolinoM,LuY,FinchJ,SlatopolskyE,DussoAS.p21WAF1 andTGF-alphamediateparathyroidgrowtharrestbyvitamin Dandhighcalcium.KidneyInt.2001;60:2109–17.
40.CanaffL,HendyGN.Humancalcium-sensingreceptorgene. VitaminDresponseelementsinpromotersP1andP2confer transcriptionalresponsivenessto1,25-dihydroxyvitaminD.J BiolChem.2002;277:30337–50.
41.ChapuyMC,PreziosiP,MaamerM,ArnaudS,GalanP, HercbergS,etal.PrevalenceofvitaminDinsufficiencyinan adultnormalpopulation.OsteoporosInt.1997;7:
439–43.
42.CranneyA,HorsleyT,O’DonnellS,WeilerH,PuilL,OoiD, etal.EffectivenessandsafetyofvitaminDinrelationtobone health.EvidRepTechnolAssess.2007;158:1–235.
43.KuchukNO,VanSchoorNM,PluijmSM,ChinesA,LipsP. VitaminDstatus,parathyroidfunction,boneturnover,and BMDinpostmenopausalwomenwithosteoporosis:global perspective.JBoneMinerRes.2009;24:693–701.
44.Bischoff-FerrariHA,KielDP,Dawson-HughesB,OravJE,LiR, SpiegelmanD,etal.Dietarycalciumandserum
25-hydroxyvitaminDstatusinrelationtoBMDamongU.S. adults.JBoneMinerRes.2009;24:935–42.
45.ElMaghraouiA,OuzzifZ,MounachA,RezqiA,AchemlalL, BezzaA,etal.HypovitaminosisDandprevalent
asymptomaticvertebralfracturesinMoroccan
postmenopausalwomen.BMCWomensHealth.2012;12:11.
46.HolickMF,BinkleyNC,Bischoff-FerrariHA,GordonCM, HanleyDA,HeaneyRP,etal.Evaluation,treatment,and preventionofvitaminDdeficiency:anEndocrineSociety clinicalpracticeguideline.JClinEndocrinolMetab. 2011;96:1911–30.
47.AdamsJS,KantorovichV,WuC,JavanbakhtM,HollisBW. ResolutionofvitaminDinsufficiencyinosteopenicpatients resultsinrapidrecoveryofbonemineraldensity.JClin EndocrinolMetab.1999;84:2729–30.
48.Bischoff-FerrariHA,Dawson-HughesB,WillettWC,Staehelin HB,BazemoreMG,ZeeRY,etal.EffectofvitaminDonfalls:a meta-analysis.JAMA.2004;291:1999–2006.
49.Bischoff-FerrariHA,WillettWC,WongJB,GiovannucciE, DietrichT,Dawson-HughesB.Fracturepreventionwith vitaminDsupplementation:ameta-analysisofrandomized controlledtrials.JAMA.2005;293:2257–64.
50.VandenBerghJP,BoursSP,VanGeelTA,GeusensPP.Optimal useofvitaminDwhentreatingosteoporosis.CurrOsteoporos Rep.2011;9:36–42.
51.KuwabaraA,TsugawaN,TanakaK,FujiiM,KawaiN,Mukae S,etal.ImprovementofvitaminDstatusinJapanese institutionalizedelderlybysupplementationwith800IUof vitaminD(3).JNutrSciVitaminol.2009;55:453–8.
52.VonRestorffC,Bischoff-FerrariHA,TheilerR.High-doseoral vitaminD3supplementationinrheumatologypatientswith severevitaminD3deficiency.Bone.2009;45:
747–9.
53.VanGroningenL,OpdenoordtS,VanSorgeA,TeltingD, GiesenA,DeBoerH.Cholecalciferolloadingdoseguideline forvitaminD-deficientadults.EurJEndocrinol.
2010;162:805–11.
54.SandersKM,StuartAL,WilliamsonEJ,SimpsonJA,Kotowicz MA,YoungD,etal.Annualhigh-doseoralvitaminDandfalls andfracturesinolderwomen:arandomizedcontrolledtrial. JAMA.2010;303:1815–22.
55.GriffinMD,LutzWH,PhanVA,BachmanLA,McKeanDJ, KumarR.Potentinhibitionofdendriticcelldifferentiation andmaturationbyvitaminDanalogs.BiochemBiophysRes Commun.2000;270:701–8.
56.VanEttenE,MathieuC.Immunoregulationby
1,25-dihydroxyvitaminD3:basicconcepts.JSteroidBiochem MolBiol.2005;97:93–101.
57.HelmingL,BöseJ,EhrchenJ,SchiebeS,FrahmT,GeffersR, etal.1alpha,25-DihydroxyvitaminD3isapotentsuppressor ofinterferongamma-mediatedmacrophageactivation. Blood.2005;106:4351–8.
58.CutoloM.VitaminDandautoimmunerheumaticdiseases. Rheumatology.2009;48:210–2.
59.SongGG,BaeSC,LeeYH.AssociationbetweenvitaminD intakeandtheriskofrheumatoidarthritis:ameta-analysis. ClinRheumatol.2012;31:1733–9.
60.AlsOS,RiisB,ChristiansenC.Serumconcentrationof vitaminDmetabolitesinrheumatoidarthritis.Clin Rheumatol.1987;6:238–43.
61.SainaghiPP,BellanM,CardaS,CeruttiC,SolaD,NervianiA, etal.HypovitaminosisDandresponsetocholecalciferol supplementationinpatientswithautoimmuneand non-autoimmunerheumaticdiseases.RheumatolInt. 2012;32:3365–72.
62.CutoloM,OtsaK,LaasK,YprusM,LehtmeR,SecchiME,etal. Circannualvitamindserumlevelsanddiseaseactivityin rheumatoidarthritis:northernversussouthernEurope.Clin ExpRheumatol.2006;24:702–4.
63.FairneyA,StraffenAM,MayC,SeifertMH.VitaminD metabolitesinsynovialfluid.AnnRheumDis.2014; 46.
64.MawerEB,WoolleyDE.VitaminDreceptorsintherheumatoid lesion:expressionbychondrocytes,macrophages,and synoviocytes.AnnRheumDis.1999;58:118–21.
65.ZwerinaK,BaumW,AxmannR,HeilandGR,DistlerJH, SmolenJ,etal.VitaminDreceptorregulatesTNF-mediated arthritis.AnnRheumDis.2011;70:1122–9.
66.VanHamburgJP,AsmawidjajaPS,DavelaarN,MusAM, CornelissenF,VanLeeuwenJP,etal.TNFblockaderequires 1,25(OH)2D3tocontrolhumanTh17-mediatedsynovial
inflammation.AnnRheumDis.2012;71:606–12.
67.KerrGS,SabahiI,RichardsJS,CaplanL,CannonGW,Reimold A,etal.PrevalenceofvitaminDinsufficiency/deficiencyin rheumatoidarthritisandassociationswithdiseaseseverity andactivity.JRheumatol.2011;38:53–9.
69.SainaghiPP,BellanM,NervianiA,SolaD,MolinariR,Cerutti C,etal.Superiorityofahighloadingdoseofcholecalciferolto correcthypovitaminosisDinpatientswith
inflammatory/autoimmunerheumaticdiseases.JRheumatol. 2013;40:166–72.