w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Body
composition
of
Fanconi
anemia
patients
after
hematopoietic
stem
cell
transplantation
Priscilla
Peixoto
Policarpo
da
Silva,
Daniella
Schmit,
Carmem
Bonfim,
Denise
Johnsson
Campos,
Estela
Iraci
Rabito,
Regina
Maria
Vilela
∗HospitaldeClínicasdaUniversidadeFederaldoParaná(HC/UFPR),Curitiba,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received31March2017 Accepted9June2017 Availableonline28July2017
Keywords:
Fanconianemia Bodycomposition
Hematopoieticstemcelltransplant
a
b
s
t
r
a
c
t
Introduction:Fanconianemiaisararegeneticdiseaselinkedtobonemarrowfailure;a possi-bletreatmentishematopoieticstemcelltransplantation.Changesinthenutritionalstatus ofFanconianemiapatientsarenotverywellknown.Thisstudyaimedtocharacterizebody compositionofadult,childrenandadolescent patientswithFanconianemiawhowere submittedtohematopoieticstemcelltransplantationornot.
Methods:Thiscross-sectionalstudyenrolled63patients(29adultsand34childrenand ado-lescents).Bodycompositionwasassessedbasedondiversemethods,includingtricepsskin fold,armcircumference,armmuscleareaandbioelectricalimpedanceanalysis,asthere isnoestablishedconsensusforthispopulation.Bodymassindexwasalsoconsideredas referenceaccordingtoage.
Results:Almosthalf(48.3%)ofthetransplantedadultpatientswereunderweight consider-ingbodymassindexwhereaseutrophicstatuswasobservedin66.7%ofthechildrenand adolescentssubmittedtohematopoieticstemcelltransplantationandin80%ofthosewho werenot.Atleast50%ofallgroupsdisplayedmusclemassdepletion.Halfofthetransplanted childrenandadolescentspresentedshort/veryshortstatureforage.
Conclusion:Allpatientspresentedlowmusclestores,underweightwascommoninadults, andshortstaturewascommoninchildrenandadolescents.Morestudiesareneededto detectwhethermusclemassloss measuredattheearlystages oftreatment resultsin higherriskofmortality,consideringtheimportanceofmusclemassasanessentialbody componenttopreventmortalityrelatedtoinfectiousandnon-infectiousdiseasesandthe malnutritioninherenttoFanconianemia.
©2017Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthorat:UFPR,JardimBotânicoCampus,DepartmentofNutrition,AvPrefeitoLothárioMeissnern◦3400,JardimBotânico,
80050-540Curitiba,PR,Brazil.Tel.:+5541991115454. E-mailaddress:regina.vilela@mail.mcgill.ca(R.M.Vilela).
http://dx.doi.org/10.1016/j.bjhh.2017.06.004
Introduction
Fanconianemia(FA)isararerecessivegeneticdisease, usu-allyinherited inanautosomal recessive manner,linked to bonemarrowfailure andanincreasedriskofdeveloping a tumor.1FApatientsaremorepronetopresenting
morpholog-icalandendocrineabnormalities,suchasbonedeformities, cardiac and kidney malformation, hyperpigmentation, glu-coseintolerance,changesinglycemiccontrol,dyslipidemia, hypothyroidismandgrowthhormonedeficiency.2
Theonlyhematologytreatmentthatoffersapotentialcure forthis disorderishematopoieticstemcell transplantation (HSCT),whichaimstorestoretheimpairedbonemarrow.1–3
SomeconditionsmayaffectthesuccessrateofHSCT,such asthestageofthedisease,thetypeoftransplant,theorigin ofdonorstemcellsandhistocompatibility,theconditioning regimen,age, previoustreatment, and nutritional statusof thepatient.4Moreover,immunosuppressionandthetoxicity
oftheconditioningregimenhavenegativeeffects,in partic-ularinrespecttoinfections,bleeding,constipation,diarrhea, mucositis,nausea,andemesis.3
Inadditiontothecharacteristicoutcomesofthedisease, suchasshortstature,transplantedpatientswithFAcanbe underweight, and present increased growth problems and dyslipidemia,whereaschangesinglycemicandhormone con-trolmayresultinanoverweightconditionorevenobesity.5
Malnutrition has substantial prognostic and socioeco-nomicimplicationsforpatientsandcaregivers.Consequences ofmalnutritionincludeincreasedcomplicationsafter surger-iesandprolongedhospitalizationresultinginhigherexposure toinfectiousagents,areducedresponsetotreatment,apoorer qualityoflifeandultimatelyaworseprognosis.Individually, methods ofnutritional assessmentare limited and a gold standardhasnotbeenestablishedtodate.Consequently,at leasttwoinstrumentshavebeenusedtoestablishthe nutri-tionaldiagnosisadequately.6Inthecaseofdiseasesthathave
complexmetabolicdemandandrequireadrugregimenthat substantiallyaffectcellstructureandimposecatabolismsuch aschemotherapy,theattentiontonutritionalstatusshouldbe stressed,however,thecriteriatochoosethemostadequate methodshavenotbeenclearlydefined.7
AsFApatientsaremorepronetomalnutritionnotonlydue totreatmentbutalsobecauseofthemetabolicburdenrelated tothedisease,andastheymighthavehigherriskofmortality, thisstudyinvestigatedwhetherchangesinbodycomposition areevidentaftertransplantationandwhetherthereare dif-ferencesbetweenpatientssubmittedtotransplantandthose whoarenot.Therearefewstudiesthatanalyzethenutritional statusofthesepatients,whichmakesitdifficultto discrimi-natethisgroupfrompatientswithhematologicaldiseasesand asaconsequence,duringtheclinicalpractice,nutrition sup-portmaynotbespecifiedtoachievetheirneedsresultingin chronicmalnutrition.Studiestobettercharacterizethe nutri-tionalstatusofFApatientsafterHSCTareneededtooptimize supportivecareinthisuniquepopulation.
Thepresentstudyaimedtocharacterizethebody compo-sitionofchildren,adolescentsandadultpatientswithFAone yearorlongerafterHSCTascomparedtopatientsundergoing
clinicaltreatmentinordertosupportfuturestudiesrelatedto nutritionalassessment.
Methods
This cross-sectional study was conducted with male and femaleFApatientswhoweretwoyearsofageorolderand sub-mittedtoHSCTornotattheBoneMarrowTransplantService (BMTS)oftheHospitaldeClínicasdaUniversidadeFederaldo Paraná. Exclusioncriteriawerepatientsthat hadbeen sub-mittedtoHSCTwithinsixmonthsofthestartofthisstudy, presenceofphysicalchangesthatcouldimpair anthropomet-ricassessments,andcognitivedifficultiestoread,understand andfilloutquestionnaires.Tocharacterizethepopulation,a structuredquestionnairewasusedconsistingofidentification data(dateofbirthandgender)andclinicaldata(submission toandtypeofHSCT,timeafterHSCT,andcomorbidities).
ThisstudywasapprovedbytheHumanResearchEthics CommitteeoftheHospitaldeClínicasdaUniversidadeFederal doParaná(#347232140.0.0000.0096),andawrittenconsentwas obtainedfromallparticipantsortheirlegalguardians.
Anthropometricassessment
Weight,height,armcircumference(AC)andtricepsskinfold (TSF) were measured,and bodymassindex (BMI)and arm musclearea(AMA)werethencalculated.The15thpercentile wasusedasacut-offpointforinadequacy.8Foradultpatients
(≥19 years old) BMI was classified according to the World Health Organization.9 Toclassifychildrenand adolescents,
theWHOAntro®programwasusedforchildrenyoungerthan fiveyearsold,andtheWHOAntroPlus® programwasused forthoseagedbetweenfiveandlessthan19yearsold.Z-score valueswereusedtoclassifyweightforage(W/age),heightfor age(H/age),andBMIforage(BMI/age)ratios.10
Bodycompositionandphaseangleassessment
Bioelectricalimpedanceanalysis(BIA)wasperformedusinga tetrapolarBIA deviceQuantum101(RJLSystem®, Inc.USA) with a current of 800A and frequency of50 KHz. It was
appliedaccordingtothe instructionsprovidedbythe guide forbioelectricalimpedanceanalysis.11Phaseanglewas
calcu-latedfromthearctangentresistance/reactance(Xc/R)value, which was expressed in degrees after being multiplied by 180/.12Phaseanglestandardizationwasbasedonthe
follow-ingequation:observedphaseangle(◦)
−meanphaseanglefor genderandBMI(◦)/phaseanglestandarddeviationforgender
andBMI.13
AsthereisnoreferenceforphaseanglestratifiedbyBMI under18.5kg/m2,18individualswerenotconsideredinthis
analysis.Foradults,phaseanglevaluesbelow5◦were
consid-eredriskpredictorsformalnutritionandmorbidity.14
agecriteriawerenotincludedinthisanalysis.15All
measure-mentswere performed atonetimepointand therewasno follow-up.
Statisticalanalysis
TheIBM® Statistical Packageforthe SocialSciences (SPSS) version22.0wasusedtoperformstatisticalanalyses. Demo-graphic and anthropometric variables were evaluated by descriptivestatistics.TheShapiro–Wilktestwasusedtoverify thehomogeneityofdata.Sensitivityandspecificitywas deter-minedbythe receiveroperatorcharacteristics (ROC) curve, consideringnutritionalstatus(NS)andthetimeafterHSCT. Student’st-testandMann–WhitneyUtestwereusedto com-parethemeansofindependentsamples;bothtestswereused tocomparetransplantedandnon-transplantedchildrenand adolescents.A p-value <0.05 (95% confidenceinterval) was usedtoidentifysignificantdifferencesbetweenthegroups.
Results
Demographiccharacteristics
Sixty-eight patients were recruited but five were excluded due to the exclusion criteria thus the total study popula-tionwas63patients–32maleand31female.Childrenand adolescentswereagedbetweentwoand18yearsold(mean: 13.1±3.85) whileadultswereagedfrom 19 to40years old (mean:25.0±5.16).
Mostpatients(84%–n=53)underwentHSCT.Allogeneic relateddonorHSCTweremostcommoninadults(69%–n=20) andinchildrenandadolescents(66.7%–n=16). Haploiden-ticaltransplantswerethemostcommon typeoftransplant (62.5%–n=10)forchildrenandadolescents.Thetimeafter HSCTrangedfromsixmonthsto27 years(mean:9.0±6.75 years–Table1).Comorbiditieswerepresentin30.9%(n=21)of patientswithhypothyroidism(28.6%–n=6),hyperthyroidism (23.8%–n=5),diabetes(17.3%–n=3),andhypertension(9.5% –n=2)beingthemostcommon.
Generalbodycompositionandanthropometricassessment
Thechildrenandadolescentpopulationstudiedwas homo-geneousregardingage,anthropometricparametersandBIA assessments(Table2).Whenthedatawereclassifiedtogive clinical relevance to the findings, differences between the groupswerefoundasdescribedbelow.
Bodycomposition
Adultssubmittedtohematopoieticstemcelltransplantation
Considering anthropometric data and BMI of all patients, 48.3%(n=14)wereunderweight,followedbyeutrophic(37.9% –n=11)andoverweight(13.8%–n=4).Theaverageheightwas 1.514±0.097m.
Regardingbodycompositionaccordingtoanthropometric assessments,72.4%(n=21)hadadequateadiposetissue,58.6% (n=17)hadreducedAC,and69%(n=20)haddepletedmuscle storesaccordingtotheAMA.
The mean percentage of lean body mass (%LBM) and body fat (%BF) by the BIA assessment were 77.1±7.08% and 22.9±7.08%, respectively. The mean phase angle was 6.5◦
±1.05◦;however,only15patientshadtheirphaseangle
standardizedaccordingtopreviousestablishedcriteriawith a mean of1.2◦±1.36◦. According to the risk classification,
14.3% ofpatients presented a phase angle ofless than 5◦
(Table2).Inadults,tocontroltheinterferenceof chemother-apyregimensonnutritionalstatus,theChi-squaretestwas useddichotomizingthetreatmentprotocolsasmyeloablative versus reduced intensity conditioning (non-myeloablative) chemotherapy. Treatment did notrepresent a confounding variableformalnutritionwiththeoutcomedichotomizedas eutrophic versus undernourished according to the BMI (p -value=0.53).
Childrenandadolescentssubmittedtohematopoieticstem celltransplantation
Most childrenand adolescents who underwentHSCT were eutrophic (66.7% – n=16), considering their BMI/age ratio. However,someofthesepatientswereoverweight(8.3%–n=2), andthisgroupwastheonlyonetopresentobesepatients(8.3% –n=2).Althoughthemajoritywereeutrophicorobese,16.7% wereunderweight.RegardingtheH/ageratio,50%hadshort or veryshortstature andthe remaining50%had adequate heightsforage.
Body composition through anthropometric assessment revealedthat95.8%(n=23)ofpatientspresentedadequate adi-posetissuestoresaccordingtotheirTSF,whereas37.5%(n=9) hadreducedAC,and54.2%(n=13)haddepletedmusclestores accordingtotheAMA.
BodycompositionanalyzedbyBIAfoundmeanvaluesfor %LBMof75.2±7.79and%BFof24.8±7.79.Themeanphase angle was 6.0◦±1.04◦, and the mean standardized phase
angle,establishedin23patients,was0.7◦±1.38◦.
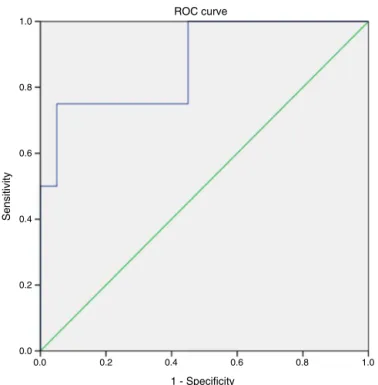
AnassessmentofNSusingtheROCcurve(Figure1)showed adiscriminatorypowerbetweenBMI/ageandtimeafterHSCT. BMI/agepresentedgreaterspecificityand sensitivityat1.56 yearsafterHSCT.
Childrenandadolescentsnotsubmittedtohematopoietic stemcelltransplantation
Most children and adolescents who were not submit-ted to HSCT were eutrophic (80% – n=8) considering BMI/age, followed by underweight in 10% (n=1), and overweight in 10% (n=1). Regarding H/age, 80% (n=8) of the patients were classified as adequate height for age, and 20% (n=2) as having very short statures for age.
Regarding the anthropometricassessment, 80%(n=8)of patients presented adequate adipose tissue stores accord-ing totheirTSF,while30%(n=3)had reducedAC,and 50% (n=5) had depleted muscle stores according to the AMA. The BIA assessment revealed mean %LBM and %BF val-uesof73.3±7.73%and26.7±7.73%,respectively.Themean phase angle was 6.1◦±1.12◦, and the mean standardized
phase angle established in eight patients was 0.9◦±0.90◦.
The anthropometric data of all groups are shown in
Table1–DemographiccharacteristicsofpatientswithFanconianemia.
Variable Adults–HSCT(n=29) Childrenandadolescents– HSCT(n=24)
Childrenandadolescents– noHSCT(n=10)
Gender–n(%)
Male 12(41.4) 13(54.2) 7(70)
Female 17(58.6) 11(45.8) 3(30)
Age(years)–mean±SD 25.0±5.13 13.7±3.18 11.7±5.00
HSCT–n(%)
Allogeneicrelateddonor 20(69) 16(66.7) – Allogeneicunrelateddonor 9(31) 8(33.3) –
Post-HSCTperiod(years)–mean±SD 13.7±5.37 3.3±2.33 –
Demographicdataarepresentedaspercentages,thecorrespondingnumberofthepopulation,mean,andstandarddeviation. HSCT:hematopoieticstemcelltransplant;SD:standarddeviation.
Table2–Bodycompositionandanthropometricassessment.
Variable AdultsHSCT n Childrenand adolescentsHSCT
n Childrenand adolescentsnoHSCT
n p-value
Age(years) 25.0(19.2–40.3) 29 13.7±3.18 24 11.7±5.00 10 0.15 Currentweight(kg) 44.4±11.08 29 39.1±12.71 24 37.1±19.91 10 0.77 W/age(Z-score) – −2.4±2.73 05 −1.8±1.57 04 0.68 Height(cm) 151.4±9.68 29 146.6(84.0–175.8) 24 141.2(81–172.4) 10 0.75 H/age(Z-score) – −1.9(−7.9–1.8) 24 −0.7(−7.6–0.2) 10 0.43 BMI(kg/m2) 19.4±3.59 29 18.5±3.54 24 18.1±3.78 10 0.76 BMI/age(Z-score) – −0.5±1.61 24 −0.4±1.34 10 0.89 AC(cm) 25.0±4.36 29 22.7±4.10 24 21.7±5.28 10 0.56 TSF(mm) 14.9±5.88 29 14.5±5.41 24 11.5±4.78 10 0.14 AMA(cm3) 33.8±10.72 29 26.9±8.44 24 27.4±11.82 10 0.92 Resistance() 772.2±129.4 28 747±147.6 24 746.5±165.88 10 0.99 Reactance() 85.5±9.57 28 76±9.88 24 76.8±10.63 10 0.85 Phaseangle(◦) 6.5±1.05 28 6.0±1.04 24 6.1±1.12 10 0.82
Standardizedphaseangle(◦) 1.2±1.36 15 0.7±1.38 23 0.9±0.9 08 0.51
Leanbodymass(kg) 34.3±6.81 23 31.4±7.42 19 36.5±12.12 06 0.36 %LBM 77.1±7.08 23 75.2±7.79 19 73.3±7.73 06 0.61 %BF 22.9±7.08 23 24.8±7.79 19 26.7±7.73 06 0.61
HSCT:hematopoieticstemcelltransplant;BMI:bodymassindex;BMI/age:bodymassindexforage;H/age:heightforage;%LBM:percentage ofleanbodymass;%BF:percentageofbodyfat;AC:armcircumference;TSF:tricepsskinfold;AMA:armmusclearea.
Parametricdatawerepresentedasaveragesandstandarddeviation,andnon-parametricdatawerepresentedasmedian,minimum,and maximum.Inordertocompareindependentgroupsthatpresenteddifferentnormalitytestsforthesameparameter,theresultswereshown asmedian,minimum,andmaximum.Statisticalsignificance:p-value<0.05.
Discussion
Inthisstudy,adultFApatientssubmittedtoHSCTpresenteda highprevalenceofunderweightaccordingtotheirBMI,and asubstantial muscle massdepletion when their AMAwas studied.When thephaseanglewas analyzed,themajority ofthe adultpatients had normal values.Most ofthe chil-drenandadolescentssubmittedtoHSCTornothadadequate bodyweightsaccordingtotheirBMI,buttheyalsohad deple-tionofmusclemassconsideringtheirAMA.Furthermore,an analysisoftheROCcurve,evenconsideringonlythe poten-tiallyhealthierpatientssincetheywereevaluatedmorethan sixmonthsafterthetransplant,showedthatmorethanone andahalfyearswouldbenecessarytoreachnormalweight parametersaccordingtotheirBMI.Studiesindicatethatfrom 22% to 38% of FA patients are underweight3,16; this may
occur due toanatomical alterations ofthe gastrointestinal tract,chronicinflammation,infection,orasconsequencesof
pharmacologicaltreatment,resultinginearlysatiety,reflux, nausea, emesis, and diarrhea.17 This study found similar
results: 20% of non-transplanted children and adolescents haddecreasedBMI.Intransplantedadultpatients,thisstudy found anevengreater prevalenceofunderweight subjects, nearlyhalfoftheadults.Incontrast,thesepatientsarealso atriskforexcessiveweightincludingobesity,inmostcases, probablyduetometabolicalterations,especially abnormali-tiesintheglucoseandinsulinmetabolismaswasobserved in studiesperformed withhumans and animals.3,18
ROC curve
Sensitivity
1 - Specificity 1.0
1.0 0.8
0.8 0.6
0.6 0.4
0.4 0.2
0.2 0.0
0.0
Figure1–Receiveroperatorcharacteristicscurve representingtimeafterHSCTversusnutritionalstatus accordingtobodymassindex/ageratio(eutrophicand undernourished).Areaoverthecurve0.875.
69%
54%
50% 48%
16.7%
10% 50%
20%
0% 10% 20% 30% 40% 50% 60% 70% 80%
Adults Submitted to HSCT Children and Adolescents Submitted to HSCT
Children and Adolescents not Submitted to HSCT
Muscle mass depletion Underweight Short/Very short stature
Figure2–Comparisonofinadequatepercentagesforarm
musclearea,bodymassindexandheight/ageratio.
Eventhoughtheanthropometricdataoftransplantedand non-transplanted patients were not statistically different, thenutritional statusreachednormalvalues, basedonthe BMI/age,only1.56yearsaftertheHSCT.Apparently,this recov-erywasnotexplainedbymusclemassconsideringthatAMA valuesshoweddepletedmusclestoresinallthepatients.The averagefatfreemass(%)foundinchildrensubmittedto trans-plantinthisstudywashigherthantheaveragefoundinour previousstudy,20however,thefirstpopulationstudiedwas
dif-ferentbecauseitwasnotexclusivelyFApatients.Thomázetal. assessed56adultpatientswhoreceivedallogeneicHSCT,and identifiedthatreducedmusclemasswasdirectlyassociated withmortalitywithin180dayspost-HSCT.Therefore,during thenutritionalassessment,BMIasanutritionalstatus predic-torshouldbeusedcarefullyasitmightunderestimatemuscle
loss.Itisworthmentioningthatchildrenandadolescents sub-mittedtoHSCTornotseemtohavehigherincreasesintotal bodymasscomparedtoadults.Inthisregard,asthisstudydid notanalyzepaireddata,nutritiontransitionmightexplainthe factthatchildrenandadolescentswereclassifiedaseutrophic, overweightandevenobese,whilemostadultswereclassified as underweight.The growingamounts ofhighlyprocessed foodeatenbyyoungpeopleshouldbeconsidered,however, foodintakeanalysiswouldbenecessarytomakethis infer-ence.
Asmostmethodsavailabletodeterminenutritional sta-tus areindirectand canbelimitingparametersastheyare influencedbynon-nutritionalfactors,thisstudyused differ-ent methods to assess nutritional status in order to have complementaryinformation.ItseemsthattheBIAmethod, whichassessesthecellmembraneandchangesinthe hydro-electrolyticbalance,canbeusedtodetectearlystructuralcell changesasaresponsetonutritioninadequacy.21Thephase
angle hasbeen studiedasan importantprognostic indica-torformanyprocessesrelatedtocellmembranedysfunction, as seeninundernutrition,acting asapredictor forclinical ornutritionalchanges.Lowphaseanglesareassociatedwith alossofcellularintegrity,22,23 whichisexpectedasaresult
ofchemotherapyand that mayworsen withverylow food intakeduringconditioningbeforeHSCTandduringthefirst twoweeksaftertheevent,whichhasalreadybeenreported inpreviousstudies.20Thephaseangledetectscellalterations
earlierthanthoseobservedbyanthropometricandlaboratory tests.24Studiesdemonstratedthatthephaseangleforhealthy
individuals can vary from 4◦ to 10◦ or from 5◦ to 15◦.25,26
Barbosa-Silvaetal.prospectivelyevaluated279patients sub-mittedtoelectivegastrointestinalsurgeryandreportedthat phaseanglesbelow5◦indicateriskformalnutritionand
mor-bidity.AfterassessingadultpatientssubmittedtoHSCT,this studyidentifiedaphaseanglegreaterthan5◦inmostcases
indicatingintegrityofbodycellmassandpreservedcell mem-branes.
Comparingthestandardphaseangleofchildrenand ado-lescentssubmittedtoHSCTornot,thecurrentstudydidnot findanysignificantdifferencesbetweenthegroups suggest-ing cell membrane recoveryin the post-HSCT period. Itis importanttoemphasizethatchangesinelectricalproperties oftissuesandmembraneionconductivityreducethephase angleinthepost-HSCTperiod,however,theelectrical prop-ertiestendtoberestoredwithnutritionalrecovery.27Infact,
Fariasetal.observedthatthephaseanglestandardcanbe a survival indicator for childrenand adolescents 180 days after HSCTwiththe currentanalysis occurringatleastsix monthsafterthetransplant.Evenconsideringthelateperiod ofassessmentinthepresentstudy,therecoveryofcellmass maynotfollowthesamepathasthedepletionofmusclemass wasfoundtobelowerthanexpectedintheadultswithFA.In fact,thepresentstudyshowedthatapproximatelyhalfofthe transplantedchildrenandadolescentpopulationpresentedZ -scoresforH/agebelowtheexpected.Theseresultsaresimilar totheonesfoundbyDenardietal.Barnumetal.28performed
anincidenceof50%.PatientswhodidnotreceiveHSCTand wereolderthan16yearsolddidnotpresentshortstature.In thisstudy,whenthatsameagerangestratificationwas per-formedrelatedtoHSCT,theincidenceofshortstaturewas 41.7%and63.6%forunder10-yearoldsandthosebetween10 and16,respectively,however,shortstaturewasnotpresentin patientsolderthan16yearsold.
Ontakingthebodycompositionandcellintegrityresults together,itisimportanttomentionthatthenutritional assess-mentshouldbeperformedcarefullysinceBMIindicatesthat mostpatientswereeutrophic,however,musclemass deple-tionispresentinatleast50%ofthecases.BMIcouldbeuseful toanalyzeweightrecovery,however,itshouldnotbetheonly parameterconsideredforthesepatients.Moreover,itseems thatthestandardphaseangleisanimportantparameterto verifycellintegritybeforetransplantasaprognosticmethod asreportedpreviously,20however,itisnotsensitivewhenit
ismeasuredsixmonthsafterthetransplant.Consideringthe importanceofmusclemassasanessentialbodycomponent topreventmortalityrelatedtoinfectiousandnon-infectious diseasesandthemalnutritionintrinsictoFA,theresultsofthis studyopenperspectivesformorestudiesfocusedonmuscle masslossandmortalityriskinFApatients.
This study presented some limitations including: the sizeofthepopulationregardingthesubgroupstratification, absence of phase angle stratified by BMI reference values fortheBrazilianpopulationresultingintheuseofreference valuesfortheGerman populationand absenceofasexual maturityassessment,whichwouldbebeneficialtoassessthe developmentofchildrenandadolescentsaswellasthe cross-sectionalcharacteristicofthestudythatdidnotallowpaired comparisonsespeciallyrelatedtobodycompositionanalysis beforeandafterHSCT.Moreover,thelackofstudiesperformed exclusivelywithFApatientslimitedthepossibilityof compar-ingtheresults.
Conclusion
AllgroupsofFApatientshadlowmusclestores,being under-weightwascommonamongadultsdiagnosedwithFA,and shortstaturewascommoninchildrenandadolescents.This study showed that the longer after the HSCT, the higher thetendencyofreachinganormalweightaccordingtothe patient’s BMI. Regular nutritional assistance is highly rec-ommended,as well as monitoring body composition, food intakeandgastrointestinalcomplicationsinordertocontrol theweightandoptimizegrowth.Morestudiesareneededto addresstheassociationbetweenmusclemasslossandriskof mortality/morbidityamongFApatients.
Conflicts
of
interests
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
Theauthorswouldlikethethanktheparticipationofthe chil-drenandadolescentsandtheirparents,aswellastheadults
along withtheBoneMarrow TransplantDepartmentofthe Hospital deClínicas da Universidade FederaldoParanáfor allowingustocompletethestudy.
r
e
f
e
r
e
n
c
e
s
1.MedeirosLA,PasquiniR.Anemiaaplásicaadquiridaeanemia deFanconi–DiretrizesBrasileirasemTransplantede Células-TroncoHematopoéticas.RevBrasHematolHemoter. 2010;32Supl.1:40–5.
2.GiriN,BatistaDL,AlterBP,StratakisCA.Endocrine abnormalitiesinpatientswithFanconianemia.JClin EndocrinolMetab.2007;92(July(7)):2624–31.
3.FumaniHK,MohammadZ,KasaeianA,AlimoghaddamK, AsadollahM,BaharB,etal.Allogeneichematopoieticstem celltransplantationforadultpatientswithFanconianemia. MediterrJHematolInfectDis.2016;8(1):e2016054.
4.EbensCL,MacMillanML,WagnerJE.Hematopoieticcell transplantationinFanconianemia:currentevidence, challengesandrecommendations.ExpertRevHematol. 2016;(December):81–97.
5.SheeanPM,BraunschweigCA.Exploringtheclinical
characteristicsofparenteralnutritionrecipientsadmittedfor initialhematopoieticstemcelltransplantation.JAmDiet Assoc.2007;107(8):1398–403.
6.MaasbergS,Knappe-DrzikovaB,VonderbeckD,JannH, WeylandtKH,GrieserC,etal.Malnutritionpredictsclinical outcomeinpatientswithneuroendocrineneoplasia. Neuroendocrinology.2017;104(1):11–25.
7.SommacalHM,JochimsAMK,SchuchI,SillaLM.Comparac¸ão demétodosdeavaliac¸ãonutricionalempregadosno
acompanhamentodepacientessubmetidosatransplantede células-troncohematopoéticasalogênico.RevBrasHematol Hemoter.2010;32(1):50–5.
8.ThomázAC,SilvérioCI,CamposDJ,KieutekaEEM,RabitoEI, FunkeVA,etal.Pre-transplantarmmusclearea:asimple measuretoidentifypatientsatrisk.SupportCareCancer. 2015;23(July(11)).
9.WorldHealthOrganization.PhysicalStatus:theuseand interpretationofanthropometry.WHOTechnicalReport Series.Geneva,Switzerland:WHO;1995,n.854.
10.WorldHealthOrganization.WHOreferences;2007.
http://www.who.int.growthref/who2007[accessed20.9.15]. 11.KyleUG,BosaeusI,LorenzoAD,DeuenbergP,EliaM,Goméz
JL,etal.BioelectricalimpedanceanalysispartII:reviewof principlesandmethods.ClinNutr.2004;23(October (5)):1226–43.
12.BaumgartnerRN,ChumleaWC,RocheAF.Estimationofbody compositionfrombioelectricimpedanceofbodysegments. AmJClinNutr.1988;50(August(2)):221–6.
13.Bosy-WestphalA,DanielzikS,DorhoferRP,LaterW,Muller MJ.Phaseanglefrombioelectricalimpedanceanalysis: populationreferencevaluesbyage,sex,andbodymassindex. JParenteralEnteralNutr.2006;30(July/August(1)):309–16.
14.Barbosa-SilvaMC,BarrosAJ,PostCL,WaitzbergDL,
HeymsfieldSB.Canbioelectricalimpedanceanalysisidentify malnutritioninperioperativenutritionassessment? Nutrition.2003;19(5):422–6.
15.HeywardVH,StolarczykLM.Avaliac¸ãodacomposic¸ão corporalaplicada.3rded.SãoPaulo:Manole;2000.
16.RoseSR,MyersKC,RutterMM,MuellerR,KhouryJC,Mehta PA,etal.Endocrinephenotypeofchildrenandadultswith Fanconianemia.PediatrBloodCancer.2012;59:690–6.
74–98.http://fanconi.org/images/uploads/other/Chapter4 Guidelines4thEdition.pdf[accessed20.2.17].
18.LiJ,SippleJ,MaynardS,MehtaPA,RoseSR,DaviesSM,etal. Fanconianemialinksreactiveoxygenspeciestoinsulin resistanceandobesity.AntioxidRedoxSignal.
2012;17(8):1083–98.
19.DenardiI,CamposDJ,RibeiroLL,PereiraCP,KieutekaEE, VilelaRM.Estadonutricionaleconsumoalimentarde pacientescomanemiadeFanconisubmetidosaotransplante decélulas-troncohematopoiéticas.RevBrasNutrClin. 2016;31(2):149–55.
20.FariasCLA,CamposDJ,BonfimCMS,VilelaRM.Phaseangle fromBIAasaprognosticandnutritionalstatustoolfor childrenandadolescentsundergoinghematopoieticstemcell transplantation.ClinNutr.2013;32(June(3)):420–5.
21.GuptaD,LisCG,DahlkSL,VashiPG,GrutschJF,Lammersfeld CA.Bioelectricalimpedancephaseangleasaprognostic indicatorinadvancedpancreaticcancer.BrJNutr. 2004;92(6):957–62.
22.GuptaD,LisCG,DahlkSL,KingJ,VashiPG,GrutschJF,etal. Therelationshipbetweenbioelectricalimpedancephase angleandsubjectiveglobalassessmentinadvanced colorectalcancer.NutrJ.2008;7(19):1–6.
23.AlvesFD,SouzaGC,ClausellN,BioloA.Prognosticroleof phaseangleinhospitalizedpatientswithacute
decompensatedheartfailure.ClinNutr.2016,
http://dx.doi.org/10.1016/j.clnu.2016.04.007.
24.LuisDA,AllerR,IzaolaO,TerrobaMC,CabezasG,CuellarL. Tissueelectricpropertiesinheadandneckcancerpatients. AnnNutrMetab.2006;50(1):7–10.
25.SilvaLMDL,CarusoL,MartiniLA.Aplicac¸ãodoângulodefase emsituac¸õesclínicas.RevBrasNutrClin.2007;22(4):317–21.
26.Barbosa-SilvaMC,BarrosAJ,WangJ,HeymsfieldSB,Pierson RN.Bioelectricalimpedanceanalysis:populationreference valuesforphaseanglebyageandsex.AmJClinNutr. 2005;82(1):49–52.
27.TyagiR,MishraS,GaurN,AwsathiRC,MisraR,JainA.Roleof bioelectricimpedancephaseangleinovarianmalignancy:a hospital-basedstudy.SaudiJHealthSci.2015;4(2):111–4.
28.BarnumJL,PetrykA,ZhangL,DeForTE,BakerKS,Steinberger J,etal.Endocrinopathies,bonehealth,andinsulinresistance inpatientswithFanconianemiaafterhematopoieticcell transplantation.BiolBloodMarrowTransplant.