rev bras hematol hemoter. 2017;39(4):360–363
w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Case
Report
Primary
imatinib
failure
rescued
by
dasatinib
and
maintained
by
reintroduction
of
imatinib
Rajiv
Kumar
∗,
Rajan
Kapoor
ArmyHospital(Research&Referral),NewDelhi,India
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received30December2016 Accepted29June2017 Availableonline26July2017
Introduction
Theavailabilityofmultipletyrosinekinaseinhibitors(TKIs)for themanagementofchronicmyeloidleukemia(CML)has cre-atedthedebateonwhichonetousefrontline.1,2Thisdebate
wouldhavebeenastraightforwardonehadtherenotbeenthe hugecostdifferencesbetweenthefirstandsecondgeneration TKIs.Theimportanceofearlyanddeepermolecularresponses ontheriskofprogressionandblast crisishasbeen proven intheDASISIONandENESTndtrialsleadingtotheapproval ofbothdasatinib andnilotinibasfirstlineTKIsalong with imatinibinboththeNationalComprehensiveCancerNetwork (NCCN)andEuropeanLeukemiaNet(ELN)guidelines.3–5
How-ever,imatinibcontinuestobethemostcommonlyusedfirst lineTKIforCML,especiallyindevelopingcountrieslikeIndia. PatientswithCMLwhoarestartedonimatinibatdiagnosis andfailtoachievetheappropriatemilestonesasdefinedinthe ELNguidelinesareshiftedtoanyoneofthesecondgeneration TKIsandthencontinuedonthesameindefinitelyuntil pro-gressiontoblastcrisisorallogeneicstemcelltransplantation.6
∗ Correspondingauthorat:DepartmentofClinicalHematology,ArmyHospital(Research&Referral),DhaulaKuan,NewDelhi110010,
India.
E-mailaddress:k.rajiv76@gmail.com(R.Kumar).
Reintroductionofimatinibafterachieving majormolecular response(MMR)withasecondgenerationTKIinapatientwith primaryimatinibfailureindicativeofahighriskdiseasewould betheoreticallyincorrectandhasnotbeenreportedinthe lit-erature.However,inthispatientthisoptionwasadopteddue tospecialcircumstanceswithsurprisingresults.
Case
report
A66-year-oldmalepresentedinSeptember2009toaprivate practitionerwithcomplaintsofdyspepsiaandwasfoundto have mild leukocytosis with a total leukocyte count (TLC) of14.8×103/L.He wasreferred toour hematology center
forevaluation.Clinicallyhehad nopositivefindingsexcept forthe tipofhisspleenbeing palpable.Aperipheralblood smearshowedashifttotheleftwithmildbasophiliabutno blasts. The leukocyte alkaline phosphate score was 10/58. A bone marrow examination showed marked granulocytic and megakaryocytic hyperplasia with panmyelosis and no increase in blasts. Karyotyping showed 20/20 metaphases
http://dx.doi.org/10.1016/j.bjhh.2017.06.006
revbrashematolhemoter.2017;39(4):360–363
361
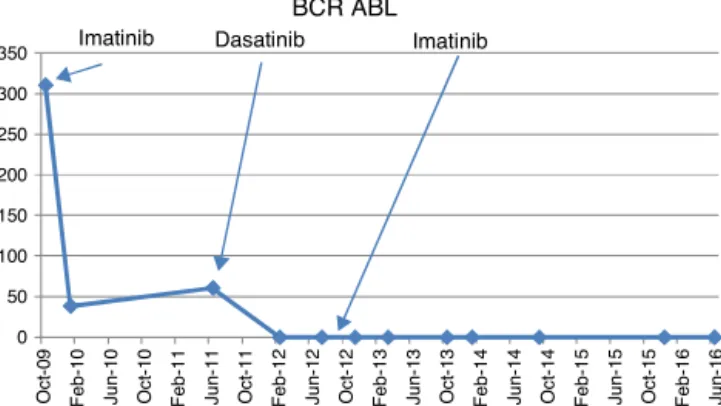
0 50 100 150 200 250 300 350 Oct -0 9 Fe b -10 J un -1 0 Oct -1 0 Fe b -11 J un -1 1 Oct -1 1 Fe b -12 J un -1 2 Oct -1 2 Fe b -13 J un -1 3 Oct -1 3 Fe b -14 J un -1 4 Oct -1 4 Fe b -15 J un -1 5 Oct -1 5 Fe b -16 J un -1 6 BCR ABLImatinib Dasatinib Imatinib
Figure1–QuantitativeBCR-ABLlevelsatdiagnosisandat follow-up.
positivefort(9;22).Aquantitativereal-timepolymerasechain reaction(RQ-PCR)forthe breakpointclusterregion-Abelson murineleukemia (BCR-ABL) waspositive forp210 at311%. Hewasstartedonimatinib(400mgOD)inOctober2009.The quantitative RQ-PCR for BCR-ABL in January 2010 at three monthswas38.56%but heachievedcomplete hematologic response(CHR).Hewasadvisedtoattendmonthlyfollow-ups. However,hefollowed upwithhislocalpractitionerand he was continued on imatinib(400mgOD) and serial RQ-PCR BCR-ABL studies were not carried out. Hereturned toour centerinJune2011whenhecontinuedtobeasymptomatic andwas inCHRbut hisRQ-PCR forBCR-ABL performedat this point was 60.71%. He claimed good compliance with imatinibtreatmentduringthis period,aswascorroborated bytheprescriptionsoftheperipheralhospital.Arepeatbone marrowkaryotypeshowedt(9;22)positivewithnoadditional cytogenetic abnormalities while a tyrosine kinase domain (TKD) mutation analysis was negative. He was started on dasatinib (100mg OD) in August 2011. The repeat RQ-PCR forBCR-ABLinFebruary 2012was0%.However,hewasnot tolerating dasatinib well with repeated episodes of loose stools, decreased appetite and abdominal discomfort. A repeatRQ-PCRforBCR-ABLinJuly2012atoneyearof dasa-tinibwas0%.Duetocontinuedintoleranceandrefusalofthe patienttotakedasatinibandnon-availabilityofnilotinib,the patientwasrestartedonimatinib(400mgOD)inAugust2012. The patient was subsequently tested by RQ-PCR for BCR-ABLonasix-monthlybasis[November2012(0.00%), March 2013(0.069%),October2013(0.062%),January2014(0.008%), September2014(0.0024%),December2015(0.00%),andJune 2016(0.00%)](Figure1).Until2014,theInternationalScale(IS) wasnotavailableforRQ-PCRinBCR-ABLmonitoringatour institution. Thereference controlgene usedwas totalABL gene.Since2015onwardstheISscaleofRQ-PCRforBCR-ABL was used for quantification. The patient has maintained ≥MMRforalmostfouryearsonimatinibpostachievementof
MMRwithdasatinibandcontinuestobeasymptomatic.
Discussion
Theconceptofachieving deepermolecularresponses with firstgenerationTKIsforasufficientperiodoftimehasbeen
thebasisforconsideringTKIwithdrawal,lookingfora pos-siblecureinCML.Thiswasdefinedasa4-logreductionof BCR-ABL1transcripts[molecularresponse(MR)4.0]formore
thantwoyearsintheStopImatinib(STIM)trialasthe crite-riaforimatinibwithdrawal.However,theresultshaveshown that60%ofpatientshadmolecularrelapsenecessitatingthe reintroductionofTKIs.Hence,deepmolecularresponsedoes notequatewithacure.7
This same concept was triedin a differentsetting ina fewcaseswheretherewasinitialimatinibfailureduetoTKD mutationsbutdeepmolecularresponseswereobtainedwith the 2nd generation TKI dasatinib. Dasatinib cessation was triedinthesecasesaswell,withno relapse.Thepresence ofBCR-ABLpositivecellsinthebloodofapatientina sta-bledrug-freeCMRappearstoindicatethateradicationofthe leukemiccloneisnotapre-requisitefortheachievementof anoperationalcureofCML.8
Giventheeconomicsof2ndgenerationTKIsindeveloping countriesandtheresultsofthesetwostudies,theoretically onemayconsiderthereintroductionofimatinibinpatients whoinitiallyfailimatinibbutdonothaveaTKDmutationand achieve deep molecular remission with dasatinib/nilotinib. Thisapproachmaybeespeciallyusefulintheresource-limited settingofdevelopingcountries,ifbackedbyadequateclinical data.However,itisimportanttonotethatrestartingimatinib canonlybeconsideredinthecontextofinitialdeepmolecular responsesbeingachievedbyasecondgenerationTKIorifthe BCR-ABLmilestonesarenotadequatelyachieved(suboptimal responses)inthefirstyearoftherapywithimatinibandare subsequentlyachievedwithsecondgenerationTKIandTKD mutationanalysisshowsnomutations.
Inourcase,therewasprimaryimatinibfailure/resistance aftercontinuedexposureforoneandahalfyearswhichwas followedbyrapidachievementofMMRwithdasatinibwithin sixmonths.AsnoTKD mutationscould bedetected,other mechanismsofimatinibresistancemighthaveplayedarole. However,thesubsequentmaintenanceofMMRwithimatinib isintriguing.
Imatinib, a phenylaminopyrimidine TKI specifically tar-gets BCR-ABL1, KIT, and PDGFR kinases.9 Dasatinib on the
other hand has amuch wider spectrum bytargeting >100 kinasesincludingBCR-ABL1,PDGFR,c-KIT,SRCfamily,EPHA family, BTK, BMX, c-FMS, etc. Moreover, dasatinib targets anearlierprogenitorpopulationthan imatiniband islikely more effective than imatinib withinthe stem cell niche.10
The mechanisms of imatinib resistance include BCR-ABL1 dependentandindependentpathways.Pharmacokinetic con-siderations, intracellularuptakeofimatinib, CMLstem cell quiescence,clonalevolutionandSRCoverexpressionarethe majorBCR-ABLindependentmechanismsforresistance. BCR-ABLoverexpressionandTKDpointmutationsleadtoBCR-ABL dependentresistance.9
Themostplausibleexplanationofthephenomenonseen in this caseappearsto bethe existenceofa predominant additionalsubclonewithdominantalternativetyrosinekinase as the driver for CML which was not affected by imati-nib but wasovercomeby dasatinib.Overexpression ofSRC kinaseisaknownmechanismofprimaryimatinibresistance and dasatinib has asignificant inhibitionof SRC kinases.9
362
revbrashematolhemoter.2017;39(4):360–363implicatedinCMLprogressiontoblast phaseand imatinib resistance.11,12Infact,LYNkinasehasbeenshowntofunction
asaregulatorofimatinibsensitivityinCML,anditisfound persistentlyactivatedinpatientsafterfailureofimatinib ther-apywhocarrynoBCR-ABL1mutations.13Theseobservations
indicatethatincellswithhighLYNexpression,acombined approachtargetingboth BCR-ABLand LYN kinases may be necessarytoovercomethisformofimatinibresistance.This canbeoneofthe explanationsofthe abovephenomenon. Onceadeepmolecularremissionwasachievedbydasatinib leadingtoneareliminationoftheclonewithdominantSRC kinase/LYN mediated proliferation, the subsequent use of imatinibwassuccessfulinmaintainingtheMMR.Inaddition, BCR-ABLdirectlyinteractswithmultipleSrcFamilyKinases (SFKs)thatsubsequentlyswitchtheAblkinaseintoanopen, activeconformationandphosphorylationoftheSH2andSH3 domainsofBCR-ABLbytheSFKsmayincreasetheactivityof theAblkinase,thusinfluencingitssusceptibilitytoimatinib.14
Hence,itcanbespeculatedthattherecouldalsobe coopera-tivetyrosinekinaseactivationamplifyingeachotherwhich wasdisruptedbythesequentialuseofimatinibanddasatinib andsubsequentimatinibreuseleadingtoMMRinthiscase.
BCR-ABL overexpression may contribute to BCR-ABL dependent resistance.6 At the onset, quantitative RQ-PCR
identifiedBCR-ABLlevelsof311%inourpatient; thiscould beanindicatorofBCR-ABLoverexpressionandcontributeto initialimatinibfailure.However,onceitwassuppressedbya morepotentTKI,dasatinib,whichhas325-foldgreateractivity againstnativeBcr-Ablcomparedtoimatinib,15the
reintroduc-tionofimatinibsuccessfullymaintainedtheMMR.
Polymutationisalso well known inCML leading toTKI resistance.9InoneofthestudiesbyQuintás-Cardamaetal.,61
patientswithCMLafterimatinibintolerance(n=10)or imat-inibresistance(n=51) whoreceived therapywithdasatinib werestudiedbyDNA expansionofspecificclonesfollowed byDNAsequencingofatleasttenclones.Thisstudy demon-stratedthepresenceof118distinctmutations(77previously notreported)involving112 aminoacids.Morethan90% of patients harbored BCR-ABL kinase domain mutations prior tothe startofdasatinib,andpolymutantsweredetectedin 57%ofpatients.16 Thetreatment ofpatientscarrying
poly-mutantsmayrequiretheuseofacombinationofTKIswith activity againstallsingle-pointmutationscontainedinthe compoundmutation.However,inourcasenoTKDmutation wasdetected.
Conclusion
IdentificationofpatientswithCMLwho could benefitwith short-termusageof2ndgenerationTKIs,eitherattheonset oronimatinibfailure,leadingtodeepmolecularresponses (≥MMR),mayhelpinshiftingthembacktoimatinibifthese responsescouldbemaintainedbyimatiniboncethehigh-risk molecular characterof the disease biology hasbeen taken careof. Thisapproach, ifproven inlarger studies,may be of considerable importance in the management of CML patientsinresource-limitedsettingswherethebenefitsofa 2ndgenerationTKImaybeextendedtoalargernumberof patients.Experiencewiththeuseof2ndgenerationTKIsin
resource-limitedsettingshasmadeitclearthatitisnot fea-sibletoimplementWesternguidelinesontheirusageintoto andrationalizationistheneedofthehoursothatmaximum benefitscanbeobtainedfromthiswonderfulclassofdrugs thathaschangedthelandscapeofCMLmanagement.
Ethical
approval
Informedconsentwas obtainedfrom all individual partici-pantsincludedinthestudy.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.MarinD.Initialchoiceoftherapyamongplentyfornewly diagnosedchronicmyeloidleukemia.HematologyAmSoc HematolEducProgram.2012;2012:115–21.
2.HughesT,WhiteD.WhichTKI?Anembarrassmentofriches forchronicmyeloidleukemiapatients.HematologyAmSoc HematolEducProgram.2013;2013:168–75.
3.KantarjianHM,ShahNP,CortesJE,BaccaraniM,AgarwalMB, UndurragaMS,etal.Dasatiniborimatinibinnewly
diagnosedchronic-phasechronicmyeloidleukemia:2-year follow-upfromarandomizedphase3trial(DASISION).Blood. 2012;119(5):1123–9.
4.HochhausA,SaglioG,HughesTP.Long-termbenefitsand risksoffrontlinenilotinibvsImatinibforchronicmyeloid leukemiainchronicphase:5-yearupdateoftherandomized ENESTndtrial.Leukemia.2016;30(5):1044–54.
5.BaccaraniM,DeiningerMW,RostiG,HochhausA,SoveriniS, ApperleyJF,etal.EuropeanLeukemiaNetrecommendations forthemanagementofchronicmyeloidleukemia:2013. Blood.2013;122(6):872–84.
6.BhamidipatiPK,KantarjianH,CortesJ,CornelisonAM, JabbourE.Managementofimatinib-resistantpatientswith chronicmyeloidleukemia.TherAdvHematol.
2013;4(2):103–17.
7.MahonFX,RéaD,GuilhotJ,GuilhotF,HuguetF,NicoliniF, etal.Discontinuationofimatinibinpatientswithchronic myeloidleukaemiawhohavemaintainedcompletemolecular remissionforatleast2years:theprospective,multicentre StopImatinib(STIM)trial.LancetOncol.2010;11(11):1029–35. 8.RossDM,BartleyPA,GoyneJ,MorleyAA,SeymourJF,Grigget AP.Durablecompletemolecularremissionofchronicmyeloid leukemiafollowingdasatinibcessation,despiteadverse diseasefeatures.Haematologica.2011;96(11):1720–2.
9.Quintás-CardamaA,KantarjianHM,CortesJE.Mechanismsof primaryandsecondaryresistancetoimatinibinchronic myeloidleukemia.CancerControl.2009;16(2):122–31. 10.CoplandM,HamiltonA,ElrickLJ,BairdJW,AllanEK,
JordanidesN,etal.Dasatinib(BMS-354825)targetsanearlier progenitorpopulationthanimatinibinprimaryCMLbutdoes noteliminatethequiescentfraction.Blood.
2006;107(11):4532–9.
11.DaiY,RahmaniM,CoreySJ,DentP,GrantS.A Bcr/Abl-independent,Lyndependentformofimatinib mesylate(STI-571)resistanceisassociatedwithaltered expressionofBcl-2.JBiolChem.2004;279(33):34227–39. 12.DonatoNJ,WuJY,StapleyJ,GallickG,LinH,ArlinghausR,
revbrashematolhemoter.2017;39(4):360–363
363
inchronicmyelogenousleukemiacellsselectedforresistance toSTI571.Blood.2003;101(2):690–8.
13.WuJ,MengF,LuH,KongL,BornmannW,PengZ,etal.Lyn regulatesBCR-ABLandGab2tyrosinephosphorylationand c-Cblproteinstabilityinimatinib-resistantchronic myelogenousleukemiacells.Blood.2008;111(7):3821–9. 14.BixbyD,TalpazM.Seekingthecausesandsolutionsto
imatinib-resistanceinchronicmyeloidleukemia.Leukemia. 2011;25(1):7–22.
15.RamirezP,DipersioJF.Therapyoptionsinimatinibfailures. Oncologist.2008;13(4):424–34.