w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Review
article
Intensity
of
anticoagulation
in
the
treatment
of
thrombosis
in
the
antiphospholipid
syndrome:
a
meta-analysis
Felipe
Freire
da
Silva
a,
Jozélio
Freire
de
Carvalho
b,∗aEscolaBahianadeMedicinaeSaúdePública,Salvador,BA,Brazil bCentroMédicodoHospitalAlianc¸a,Salvador,BA,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received19February2014 Accepted17August2014 Availableonline6January2015
Keywords:
Antiphospholipidsyndrome Thrombosis
Hemorrhage Warfarin
a
b
s
t
r
a
c
t
Introduction:Discussionabouttheintensityofwarfarininpatientswithantiphospholipid syndrome(APS)remainspresentinourdays.
Objectives: Toevaluatewhichintensityofanticoagulationwithwarfarinisassociatedwith agreaterreductionofthromboemboliceventsinthetreatmentofpatientswithAPS,aswell asassesstheriskofbleedinginthedifferenttreatmentmodalities.
Methodology:Asystematicreviewoftheliteraturewascarriedoutwithsearchfrom elec-tronic databases:PubMed,LILACSandSciELO,withtheuseofthekey-words:treatment,
warfarin,antiphospholipidsyndrome,antiphospholipidantibodysyndromeandtheirrespective translationsintoPortuguese,indifferentcombinations.Inaddition,ameta-analysiswith theaidofReviewManager5.2softwarebyCochranewasperformed.
Results:Only twoarticles metthe inclusioncriteria forthisstudy.Regarding themain outcomeassessedinthisstudy,thetwostudiesshowedsimilarvalues,indicatinghigher frequencyofthromboticeventsinhigh-intensitygroups.Thecomparativeanalysisofthe randomizedclinicaltrialevaluatedshowedanincreasedthromboticriskforthosepatients who received intervention with high-intensity warfarin. Another finding of the meta-analysiswasthehigherincidenceofminorbleeding,alsointheexperimentalgroup,that receivedwarfarinkeepingInternationalNormalizedRatio(INR)>3.
Conclusion: InindividualswithAPSandprevalenceofvenousevents,theuseofmoderate intensity(MI)anticoagulation(INR:2-3)isthemostsuitable.However,thisevidencecannot yetbeextendedtopatientswitharterialevents,duetothelimitedrepresentationofthis sampleofsubjectsinthetwoclinicaltrialsincludedinthismeta-analysis.
©2014ElsevierEditoraLtda.Allrightsreserved.
∗ Correspondingauthor.
E-mail:jotafc@gmail.com(J.F.d.Carvalho).
http://dx.doi.org/10.1016/j.rbre.2014.08.015
Intensidade
da
anticoagulac¸ão
no
tratamento
da
trombose
na
síndrome
antifosfolípide:
meta-análise
Palavras-chave:
Síndromeantifosfolípide Trombose
Hemorragia Varfarina
r
e
s
u
m
o
Introduc¸ão:adiscussãosobreaintensidadedevarfarinaempacientescomsíndrome antifos-folípide(SAF)permanecepresentenosdiasatuais.
Objetivos: avaliarqualintensidadedeanticoagulac¸ãocomvarfarinaestáassociadacom maiorreduc¸ãodeeventostromboembólicosnotratamentodepacientescomSAF,assim comoavaliaroriscodehemorragianasdiferentesmodalidadesdetratamento.
Metodologia: foirealizadaumarevisãosistemáticadaliteraturaapartirdebuscanasbases dedadoseletrônicos:PubMed,LILACSeSciELO,sendoutilizadasaspalavras-chave: treat-ment,warfarin,antiphospholipidsyndrome,antiphospholipidantibodysyndromeesuasrespectivas traduc¸õesparaoportuguês,emdiferentescombinac¸ões.Tambémfoirealizadauma meta-análisecomauxíliodoprogramaReviewManager5.2daCochrane.
Resultados: apenasdoisartigospreencheramoscritériosparainclusãonesteestudo.Em relac¸ãoaoprincipaldesfechoavaliadonestetrabalho,osdoisestudosapresentaram val-oressimilares,demonstrandomaiorfrequênciadeeventostrombóticosnosgruposdealta intensidade.Aanálisecomparativadosensaioclínicosrandomizadosavaliados demon-strouumriscotrombóticoaumentadoparaaquelespacientesquereceberamintervenc¸ão comvarfarinaemaltaintensidade.Outroachadodameta-análisefoiamaiorocorrênciade hemorragiamenortambémnogrupoexperimental,querecebeuvarfarinamantendoRazão NormalizadaInternacional(RNI)>3.
Conclusão: nos indivíduos com SAF e predominância de eventos venosos, o uso de anticoagulac¸ãoemmoderadaintensidade(MI)(RNI:2-3)estámaisindicado.Poroutrolado, essaevidênciaaindanãopodeserestendidaaospacientescomeventosarteriais,pela lim-itadarepresentac¸ãodessaamostradesujeitosnosdoisestudosclínicosincluídosnesta meta-análise.
©2014ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Antiphospholipidsyndrome(APS)isanacquiredautoimmune condition consisting of thromboembolic and/or obstetric eventsinthepresenceofcirculating antiphospholipid anti-bodies(aPLs)inplasma(anticardiolipinantibodies[aCL],lupus anticoagulant[LAC]andanti-2glycoproteinI[anti-2GPI]).1,2 Thrombosis,bothvenousandarterial,isthemostcommon clinicalmanifestationandtheonethatcausesmoremorbidity inAPS.3Venousthromboembolismispresentinabout55%of thesepatients4,mainlycharacterizedbydeepveinthrombosis (DVT)andpulmonaryembolism(PE).Themostcommon arte-rialthromboticmanifestationsarecerebrovascular accident (CVA)andtransientischemicattack(TIA),affecting approxi-mately50%ofpatientswithAPS.1,4,5
ThetreatmentofAPSthatiscurrentlyappliedincludes: (1)antiplateletagents(aspirinorclopidogrel);(2)low molecu-larweightheparinand(3)warfarin,6thusnotdifferingfrom thetreatment offeredtothegeneralpopulation presenting thromboticevent.7
In the management of patients on anticoagulant med-ication, a strict monitoring is essential in order to reach therapeuticdosesanddonotcauseadverseeffects.8AnINR between2and 3presentedbypatientsonwarfarin reflects anticoagulanttreatmentofmoderateintensity(MI),whichis themostusedandrecommendedinthescientificliterature.9 However, an INR> 3, which represents high-intensity
treatment (HI), is indicated by some previous work as the bestoptioninsomecases,insecondaryprophylaxisof thrombosis in APS.7,10–12 Most of these studies is partially based on retrospective cohort suggesting increased riskof recurrentthrombosisinpatientsonMIwarfarintherapyas comparedtotreatmentofHI.13–19 Therefore,thediscussion abouttheintensityofwarfarinforsecondaryprophylaxisof thrombosisinpatientswithAPSremainspresentnowadays.
Anothercontroversialissue,whenarticlescomparingthe twointensitiesofwarfarin(MIversusHI)inthetreatmentof patientswithpresenceofaPLsareanalyzed,istheoccurrence ofbleeding,oneofthemostdreadedcomplicationsof antico-agulanttherapythathasafrequencyof2%-3%peryear(major bleeding),similartothatofpatientswithoutAPSalso under-goinganticoagulation.20Thereisastrongcorrelationbetween theintensityofanticoagulationandtheincidenceofbleeding events.Infact,Levineetal.21saythatwealreadyhavegood evidencethatthetreatmentwithvitaminKantagonists(eg. Warfarin)withINRbetween2-3isassociatedwithlowerrates ofbleedingwhencomparedtotreatmentofmajorintensity (INR>3).Thus,whenevaluatingthereductionofthrombotic events withanticoagulanttreatment,the associatedriskof bleedingcomplicationsshouldalsobeconsidered.21
oftheseindividuals,reducingratesofmorbidityand mortal-ity,mainlyrepresentedbythefrequencyofthromboticevents andcomplications,suchasbleeding.
Therefore, the aimofthis study was toevaluatewhich intensityofanticoagulationwithwarfarin (conventional/MI vs.HI)isassociatedwithgreaterreductionofthromboembolic eventsinthetreatmentofpatientswithAPS.Asasecondary endpoint,theriskofbleedingaccordingtothedifferent inten-sitiesofanticoagulationwillbeassessed.
Methodology
Studydesign
Systematicreviewofliteratureandmeta-analysis.
Searchstrategy
A search was performed in electronic databases: PubMed, LILACS and SciELO, covering the period from 1983 (when APSwas described)to April2013.The followingkey-words wereused:treatment,warfarin(WisconsinAlumniResearch Foundation), antiphospholipid syndrome, antiphospholipid antibody syndrome and their respective translations into Portuguese,indifferentcombinations.Thereferences ofall selectedarticleswerealsoevaluatedinsearchforworkthat wasnotidentifiedintheinitialsearch.Therewerenolanguage restrictions.
Inclusionandexclusioncriteria
Scientificpapersthathavethedesignofarandomizedclinical trial(RCT)wereselectedtoassesstheuseofwarfarinfor sec-ondaryprophylaxisofthrombosisinAPSinpatientsolderthan 18years.Anyotherstudytypewasexcludedfromthisreview, aswellassubgroupanalyzesofrandomizedclinicaltrials.
Thestudieshadto:(1)presentinterventionswithwarfarin carriedout in accordance withthe conventionaltreatment (INR:2-3)andwithhigh-intensitytreatment(INR:3.1to4.5); (2)haveeachtherapycomparedwithplacebo/controlgroup orcomparedtoeachother(conventional/MIvs.HI);(3)assess asprimaryendpointtheoccurrenceofrecurrentthrombotic eventsandbleeding,and(4)classifybleedingastotal,major andminor.
Patientsselectedforthestudiesparticipatinginthisreview shouldalsomeetSapporo22and/orSydneycriteria23forthe diagnosisofAPS.Theformerincludelaboratory determina-tions of anticardiolipin of immunoglobulin G (IgG) and/or M(IgM)subtypes,and LACinpatientswitharterial/venous thrombosisorepisodeoffetalloss.Sydneycriteriarequireat leastoneclinicalandonelaboratorycriterion(involvingthe presenceofanti-2-GPIIgGand/orIgMsubtypes).
Dataselection
Thetwoauthorsofthisarticleconductedasearch individu-allyanddecidedonconsensus(accordingtopredetermined methodology)fortheselectionofitemsparticipatinginthis review.
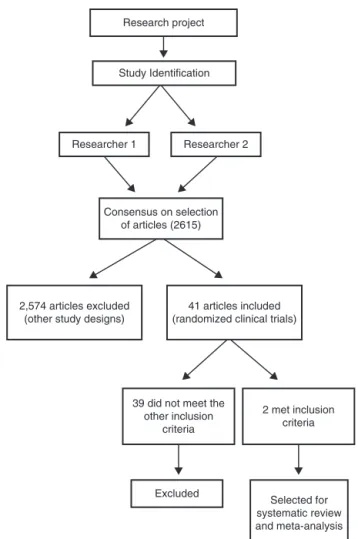
Research project
Study Identification
Researcher 1 Researcher 2
Consensus on selection of articles (2615)
2,574 articles excluded (other study designs)
41 articles included (randomized clinical trials)
39 did not meet the other inclusion
criteria
2 met inclusion criteria
Excluded
Selected for systematic review and meta-analysis
Figure1–Flowchartofmethodologyadoptedinthe selectionofstudiesincludedinthisreview.
Fig.1summarizesthemethodologyfollowedinthis sys-tematicreviewandmeta-analysisfortheselectionofstudies.
Studiesqualitativeevaluation
Thescientific papersselected were alsosubjected to qual-itative evaluationthrough the application ofJadad scale.24 Studiesthathadgrade3orgreaterontheJadadscalewere characterizedasofgoodquality. Inordertostrengthenthe assessment ofthe methodological quality ofstudies to be includedinthereview,thescaleofDowns&Blackwasalso applied.25Thismethodconsistsofaquestionnairecontaining 27 items. It evaluates: information,external validity, inter-nal validity - bias, confounding (selection bias) and study power. For each question, the article may receive a score of 0 or 1, with the exception of question 5, which can generate 2 points. Each item can get a maximum of 28 points.
Statisticsanalysis
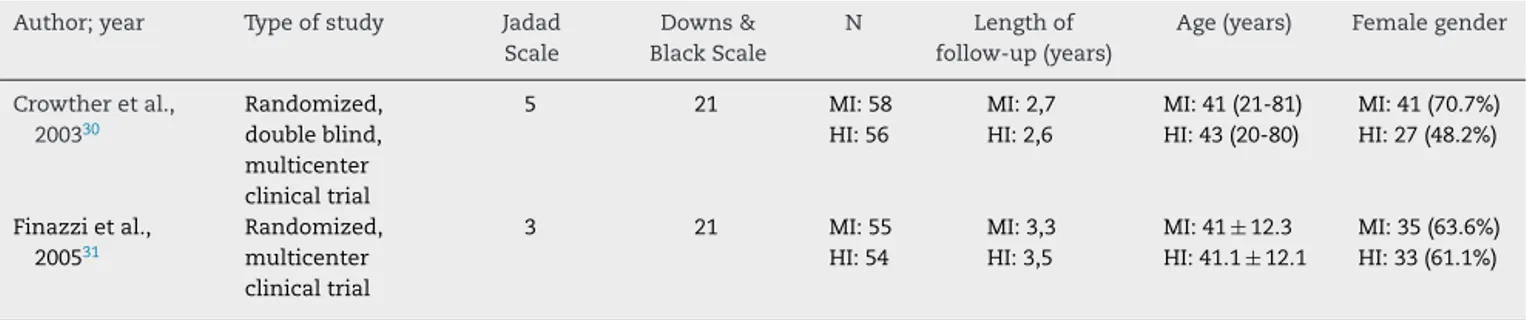
Table1–Characteristicsofstudiesevaluatingtheuseofwarfarinforsecondaryprophylaxisofthrombosisinpatients withAPS,fromINRofmoderateandhighintensity.
Author;year Typeofstudy Jadad
Scale
Downs& BlackScale
N Lengthof
follow-up(years)
Age(years) Femalegender
Crowtheretal., 200330
Randomized, doubleblind, multicenter clinicaltrial
5 21 MI:58
HI:56
MI:2,7 HI:2,6
MI:41(21-81) HI:43(20-80)
MI:41(70.7%) HI:27(48.2%)
Finazzietal., 200531
Randomized, multicenter clinicaltrial
3 21 MI:55
HI:54
MI:3,3 HI:3,5
MI:41±12.3 HI:41.1±12.1
MI:35(63.6%) HI:33(61.1%)
HI,highintensity;MI,moderateintensity;N,numberofparticipants;INR,internationalnormalizedratio;APS,antiphospholipidsyndrome.
usedwastheclassicalMantel-Haenszel.27–29Thefixed-effect modelwaschosenasanalysismodel,andtheriskratioasa measureofeffect.Ap-valueoflessthanorequalto0.05was consideredasstatisticallysignificant,withtheadoptionofa confidenceinterval(CI)of95%.
Results
Twoarticlesmettheinclusioncriteriaforthisstudy.Bothare randomizedtrials that addressed the intensity ofwarfarin usedinthetreatmentofpatientswithAPSandwerepublished insequenceintheyears2003and2005.30,31
Both studies, by Crowther et al.30 and Finazzi et al.,31 includedinthisanalysis,scored21onDowns&Blackscale,25 correspondingtomorethan70%ofthequestions,therefore suggestingstudiesofgood methodologicalquality. Further-more,thoseselectedclinicaltrialshadgrade3orgreateron Jadadscale,24alsoclassifyingtheincludedwork asofgood quality.
Themaincharacteristics ofthe studiesincluded inthis review, including its methodological evaluations, number ofparticipants, and demographic dataare summarizedon
Table1.
Although the beginning of data collection was almost simultaneous,thefollow-uptimewasslightlyhigherinthe Europeanstudy (Finazzi),31 being held for 3.5years in the groupofHIand3.3yearsforthegroupwithconventional treat-ment.TheCanadianstudy(Crowther)30presented2.7and2.6 yearsoffollow-up,respectively,forgroupsofMIandHI.
The studies showed a similar number of participants (Crowtheretal.30=114;Finazzietal.31=109),thatwere prop-erly randomized into two groups: those who received HI warfarintherapy(withanINRof3-4.5forFinazzietal.31and of3.1-4 for Crowther et al.30)and those who would be in theMIgroupwithanINRbetween2-3.However,theclinical trialbyFinazzietal.31wasjustblindonoutcomesevaluation, asopposedtoCrowtheretal.,30whichwasdouble-blinded, decreasingpotentialbiasesconsiderably.
Both studies also showed as limitation the premature discontinuationofclinicalcarewhentheHIgrouphad sig-nificantlyhigherratesofthromboticeventscomparedtothe controlgroup.
TheRCTbyCrowtheretal.30recruitedtheirpatientsfrom tertiarycareclinicsofrheumatologyandthromboembolism andhadasoneofitsinclusioncriteriapatientswithpositivity
foraPLs (LACand/oraCL) andconfirmedhistory ofarterial and/or venousthrombosis. Onthe other hand,the RCTby Finazzietal.31selectedtheirpatientsfrom26centersinfour EuropeancountriesandArgentina.InlinewiththeCanadian study,theirinclusioncriteriaweresimilar.
Regardingthecharacteristicsofthepopulationevaluated inthestudiesincludedinthisreview,theaverageagewas sim-ilar(Crowtheretal.30=42years;Finazzietal.31=40,5years). However, despite the similar percentage ofwomen in the Canadianstudy30thereisamajordisparityamong random-izedgroups(MI:71%ofwomen;HI:48%ofwomen).Noneof the studies providedinformationabout the patients’ color, butprobablythemajorityiscertainlywhite,duetotheplaces wherethescientificworkwascarriedout.
WhenevaluatingthestudybyCrowtheretal.,30something thatcalledtheattentionwasalargepercentageofpatients who left the study.31 There was only8.2% of this typeof losstofollow-up.However,therewerenodeathsinthefirst study, except for the European RCT,31 which reported five deaths.
Analysisofprothrombintime,fromINR,tocontrol treat-ment,wasobservedinbothstudies,which showedsimilar mean values(Crowther et al.30=3.3HI and 2.3MI; Finazzi etal.31=3.2HIand2.5MI).
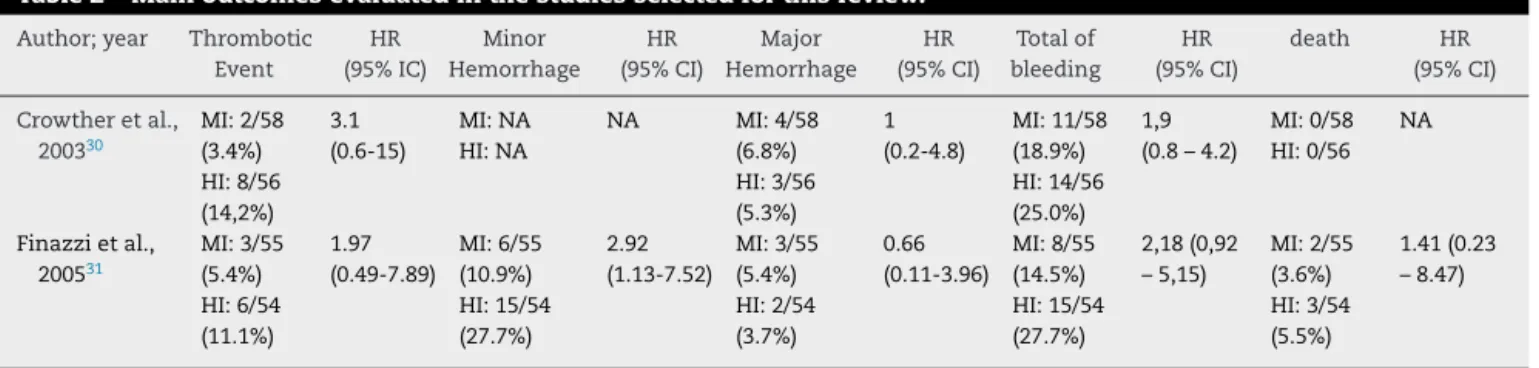
Regarding the main endpoint assessed in this study, the two studies showed similar values, indicating higher frequency of thrombotic events in HI groups (Crowther etal.30=14.2%AIvs.3.6%MI;Finazzietal.31=11.1%HIvs.5.5% MI).
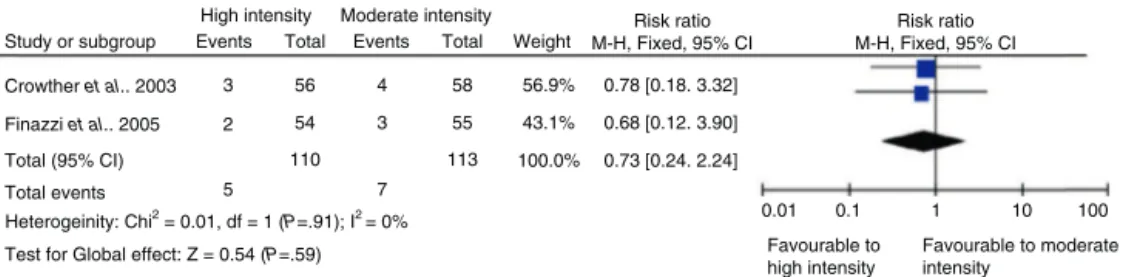
Asasecondaryendpointobservedinanticoagulant ther-apy,thefrequencyofbleedingwasevaluated.Finazzietal.31 definedmajor bleedingasonethatrequiredtransfusion or surgery,fatal,retroperitonealorintracranialhemorrhage.All othertypesofbleedingwereclassifiedasminorhemorrhage. Hemorrhage,intotal,appearedin25%ofHIvs.19%ofMIinthe Canadianstudy,30andin27.8%ofHIvs.14.6%ofMIinthe Euro-peanstudy.31Themajorhemorrhagiceventwasalsoassessed separately,beingpresentin5.3%ofHIvs.6.8%ofMI(Crowther etal.30)andin3.7%ofHIvs.5.5%ofMI(Finazzietal.31).Finally, thepresenceofminorhemorrhageintheEuropeanstudy31 washigherintheHIgroup-27.8%ofHIvs.10.9%ofMI-and wasnotseparatelyevaluatedintheCanadianwork.30
Table2summarizesthemainendpointsevaluatedinthe studiesinvolvedinthissystematicreview.
Table2–Mainoutcomesevaluatedinthestudiesselectedforthisreview.
Author;year Thrombotic
Event HR (95%IC) Minor Hemorrhage HR (95%CI) Major Hemorrhage HR (95%CI) Totalof bleeding HR (95%CI) death HR (95%CI)
Crowtheretal., 200330 MI:2/58 (3.4%) HI:8/56 (14,2%) 3.1 (0.6-15) MI:NA HI:NA
NA MI:4/58
(6.8%) HI:3/56 (5.3%) 1 (0.2-4.8) MI:11/58 (18.9%) HI:14/56 (25.0%) 1,9 (0.8–4.2)
MI:0/58 HI:0/56
NA
Finazzietal., 200531 MI:3/55 (5.4%) HI:6/54 (11.1%) 1.97 (0.49-7.89) MI:6/55 (10.9%) HI:15/54 (27.7%) 2.92 (1.13-7.52) MI:3/55 (5.4%) HI:2/54 (3.7%) 0.66 (0.11-3.96) MI:8/55 (14.5%) HI:15/54 (27.7%) 2,18(0,92 –5,15) MI:2/55 (3.6%) HI:3/54 (5.5%) 1.41(0.23 –8.47)
HI,highintensity;CI,confidenceinterval;HR,hazardsratio;MI,moderateintensity;NA,notassessed.
those patients who received interventionwith HI warfarin comparedtothegrouprandomizedtoconventional antico-agulanttreatment.Anotherfindingofthemeta-analysiswas thehigherincidenceofminorhemorrhagealsointhe experi-mentalgroup,thatreceivedwarfarinkeepinganINR>3.
The graphs of this meta-analysis results (Forest Plot) addressingtheanalysisofoutcomes-thromboticevents,total bleeding,majorbleeding,minorbleedinganddeath-are illus-tratedinFigs.2–6,respectively.
Discussion
Thisstudyconductedameta-analysisofoutcomesof throm-bosisandhemorrhageofthearticlesinthescientificliterature thatevaluateddifferentintensitiesofanticoagulationinthe treatmentofthrombosisinpatientswithAPS.
Analyzing the available scientific literature on the sub-ject,itispossibletofindobservationalstudiesofprospective and retrospective cohort that have been mostly published before the RCTs evaluated in this review and, in general, showedlowerratesofrecurrentthromboticeventsinpatients receivingwarfarinwithINR>3comparedtothosereceiving anticoagulanttherapyatalowerintensity(INR<3).13–19,32
Infact,therestrospectivecohortstudybyRosoveetal.14 evaluated70patientswithAPSandconcludedthatwarfarin therapy ofintermediate/highintensitymay providegreater antithromboticprotectioncomparedtothe use ofwarfarin of low/intermediate intensity. However, this work, besides havingaretrospectivenature,includedpatientswithout diag-nosticcriteriaforAPSasthepopulationofstudy,weakening suchscientificevidence.In1995,Khamashtaet al.,13 retro-spectivelyevaluating147patientswithAPS,alsoshowed,in theirstudy,moreefficacyinpreventingrecurrentthrombotic eventsinpatientsreceivingwarfarinwithINR>3comparedto thosetreatedwithanticoagulanttherapyoflowerintensity.
Ontheotherhand,theprospectivestudybyAmesetal.33 demonstrated that HIoralanticoagulation inpatientswith APSwasnotbetterthantheconventionaltreatmentinthe sec-ondarypreventionofthrombosis.Thisworkfollowed,foreight years,67patientswithAPS,89withhereditarythrombophilia, and24withmitralvalvereplacement.
Anotherconclusionobtainedfromtheanalysisof observa-tionalstudieswasgreatertrendtorecurrenceofthrombotic eventsofthosepatientswhohadarterialevents.These indi-vidualswouldthereforehavehighercardiovascularriskand wouldrequireamoreaggressivetherapy.However,the retro-spectivestudy thatreachedthisconclusiondidnotrequire
Study or subgroup
High intensity Events
8 56 2 58 39.8% 4.14 [0.92. 18.67]
2.04 [0.54. 7.73]
2.88 [1.07. 7.71]
55 60.2%
100.0%
0.01
Favourable to high intensity
Favourable to moderate intensity
1
0.1 10 100
3
5 54
110 113
6
14 Crowther et al., 2003
Finazzi et al., 2005
Total (95% CI)
Total events
Heterogeinity: Chi2= 0.48, df = 1 (P=.49); I2 = 0% Test for Global effect: Z = 2.10 (P=.04)
Total Events Total Weight
Risk ratio M-H, Fixed, 95% CI
Risk ratio M-H, Fixed, 95% CI Moderate intensity
Figure2–Graphofmeta-analysis(ForestPlot)forcomparativeanalysisofthromboticeventsoccurrence.
58 11 56
14 57.7% 1.32 [0.66. 2.65]
1.91 [0.88. 4.13]
1.57 [0.94. 2.63]
55 42.3%
100.0%
0.01 0.1 1 10 100
8 19 54 110 113 15 29 Crowther et al.. 2003
Finazzi et al.. 2005
Total (95% CI) Total events
Test for Global effect: Z = 1.71 (P=.09) Study or subgroup
High intensity
Events Total Events Total Weight
Risk ratio M-H, Fixed, 95% CI
Risk ratio M-H, Fixed, 95% CI Moderate intensity
Favourable to high intensity
Favourable to moderate intensity
Heterogeinity: Chi2 = 0.49, df = 1 (P=.48); I2
3 56 4 58 56.9% 0.78 [0.18. 3.32]
0.68 [0.12. 3.90]
0.73 [0.24. 2.24]
55 43.1%
100.0%
0.01 0.1 1 10 100
3
7 54
110 113
2
5 Crowther et al.. 2003
Finazzi et al.. 2005
Total (95% CI) Total events
Test for Global effect: Z = 0.54 (P=.59) Study or subgroup
High intensity
Events Total Events Total Weight
Risk ratio M-H, Fixed, 95% CI
Risk ratio M-H, Fixed, 95% CI Moderate intensity
Favourable to high intensity
Favourable to moderate intensity
Heterogeinity: Chi2 = 0.01, df = 1 (P=.91); I2 = 0%
Figure4–Graphofmeta-analysis(ForestPlot)forcomparativeanalysisofmajorbleedingoccurrence.
56
11 7 58 53.6% 1.63 [0.68. 3.90]
2.55 [1.07. 6.07]
2.05 [1.11. 3.79]
55 46.4%
100.0%
0.01 0.1 1 10 100
6
13 54
110 113
15
26 Crowther et al.. 2003
Finazzi et al.. 2005
Total (95% CI) Total events
Test for Global effect: Z = 2.30 (P=.02) Study or subgroup
High intensity
Events Total Events Total Weight
Risk ratio M-H, Fixed, 95% CI
Risk ratio M-H, Fixed, 95% CI Moderate intensity
Favourable to high intensity
Favourable to moderate intensity
Heterogeinity: Chi2 = 0.51, df = 1 (P=.48); I2= 0%
Figure5–Graphofmeta-analysis(ForestPlot)forcomparativeanalysisofminorbleedingoccurrence.
1 56 1 58 33.1% 1.04 [0.07. 16.16]
1.53 [0.27. 8.79]
1.36 [0.31. 5.93]
55 66.9%
100.0%
0.01 0.1 1 10 100
2
3 54
110 113
3
4 Crowther et al.. 2003
Finazzi et al.. 2005
Total (95% CI) Total events
Test for Global effect: Z = 0.41 (P=.68) Study or subgroup
High intensity
Events Total Events Total Weight
Risk ratio M-H, Fixed, 95% CI
Risk ratio M-H, Fixed, 95% CI Moderate intensity
Favourable to high intensity
Favourable to moderate intensity
Heterogeinity: Chi2 = 0.5, df = 1 (P=.82); I2= 0%
Figure6–Graphofmeta-analysis(ForestPlot)forcomparativeanalysisofdeathoccurrence.
thefulfillingofdiagnosticcriteriaforAPSasaprerequisitefor selectionofpatients.17
Regardingthefrequencyofbleedingevents,thestudyby Ruiz-Irastorza et al.15 foundsimilar results ofmajor hem-orrhageinratstreatedwithwarfarinaccordingtoINR≥3.5. Moreover,theworkbyAmesetal.,33Khamashtaetal.,13 Derk-senetal.,16Mu ˜nozetal.18andGirón-Gonzálesetal.32showed higherratesofbleedinginpatientstreated withHI antico-agulant,withINRratesrangingfrom3-7.5atthemomentof bleeding.
However,wemustbeawareoftheimportanceof conduct-ingsystematicreviewsnowadays,astheyminimizepotential biasesduetotheirrigidmethodology,enablingthegathering ofthebestscientificevidencethatwillbethefoundationof healthcaredecision-making.Themeta-analysis,initsturn, allowsbetter assessment ofthe evidence found in a liter-aturereviewand ofapossibleheterogeneity ofthe results presented.34,35Therefore,thisfeatureallowsimproving pre-cision and accuracy in the estimate of treatment effect, increasingitsstatisticalpower.35
Oneoftheadvantagesofthisreviewwastheveryrestrictive selectioncriteria,whichallowedamoreaccurateandreliable analysisofresults.Thus,therewastheexclusionofstudies withdesignsdifferentfrom thatofrandomizedclinical tri-als, suchas casereports, case series, case-control studies, retrospective, cross-sectionaland cohort studies (the latter
was excluded for not allowing the evaluation of interven-tions).
Another benefit arising from the designof the selected studiesisthefactthatthesearemulticenterstudies,involving atotalof39clinicalcenters,includingcitiesinEurope,Canada, UnitedStatesandArgentina,whichbringsexternalvalidityto thedatafound.Furthermore,thestudybyCrowtheretal.30 presenteddouble-blinddesign,favoringfurtherrecognitionof thevalueofitsresults.
Ontheotherhand,someimportantlimitationsofthe eval-uated studies should be highlighted.Namely,the study by Finazzietal.31wasnotdouble-blind(theyusedadhoc com-mitteeofclinicalexpertsblindedtothetreatmentadopted), whichfavorsoutcomebiases.Moreover,thesamework inter-ruptedclinicaltrialearly,becauseofpatientsleavingthestudy duetoadverseeffects,orpatientorphysicianrefusaltokeep theprotocol.
Another aspect to be considered is that the study by Crowtheretal.30 failedtoanalyzethe effectivenessof war-farininthefirstthreemonthsafterthefirstthromboticevent inpatientsinvolvedinthestudy.Thislimitationwasduetothe need,setduringthestudyprotocol,toperformtwotestsfor APLswithanintervalofthreemonths.Additionally,patients withhigh riskofbleeding, suchasthose withpriorstroke, thrombocytopenia(<50,000mm3)andgastrointestinal
inthesamewaythat,inbothstudies,patientswithrecurring events,evenduringtheuseofanticoagulantprophylaxis,were excludedfromclinicaltrials.Thus,manypatientswithsevere casesofthediseasewerenotincludedinthestudies.
Itissurprisingtonotethat, inthe studybyCrowtheret al.,30 INR targets were not achievedin 43% of the time in patientsrandomizedtotheHIwarfaringroup,whatcan per-fectlyexplainthehigherincidenceofthrombosisinthisgroup thatwas“undertreated”.InthestudybyFinazzietal.,31such informationwasnotfound.Anotherpossibleexplanationfor theseresultswouldbepoorrandomization,forexample,the biased allocation of subjects, where the most severe ones couldhavebeendistributedtotheHIgroup.
Afurthernegativecharacterofbothstudies isthat they weredevelopedintendingtodemonstratethesuperiorityof anticoagulanttreatmentwithwarfarininhighdoses. How-ever,theresultspresentedintheworkbyCrowtheretal.30 showsimilaritiesbetweenbothintensitiesofanticoagulation. Additionally,thestudy byFinazzietal.31foundevenworse outcomesintheHIgroup.
The evidence found in the studies included in this reviewshouldbecarefully evaluatedin patientswith arte-rial thrombosis,sincevenousthromboembolic events were theprevalent,representingabout70%ofcasesinboth stud-ies.Therefore,asuggestionforfutureclinicalstudiesinthis areaistheuniqueinclusionofpatientswitharterialevents, consideringthattheresultsofbothpreviousstudiesalready carriedoutmaybemoreappropriatelyappliedinpatientswith venousevents.
Allthedifficultiesenumeratedshouldbemildedbecause thisisanuncommondisease.Infact,the APShasan esti-matedprevalenceof40-50casesper100,000people.36Thus, thetwoscientificpapersincludedinthismeta-analysis rep-resentthebestmedicalevidenceavailableatthetime.And yet, this evidence should be valued as prospectivestudies withlargenumbersofparticipantsandpresenting appropri-atecriteriaforselection,inclusionandexclusionareunlikely tooccur.
Inbrief,thepresentmeta-analysiscomparedtwodifferent intensitiesofanticoagulationinAPSwiththromboticevent anddemonstratedthatpatientsonHIwarfarin(INR:3-4)had morethrombotic events(although about 40% ofthisgroup were“undertreated”) andminorbleeding. Thisfinding pro-videsevidenceofusefulnessforclinicalpractice,inthesense that,inindividualswithAPSandprevalenceofvenousevents, theuseofMIanticoagulation(INR:2.0-3.0)ismoreappropriate. Moreover,suchevidencemaynotyetbeextendedtopatients witharterialevents,duetothelimitedrepresentationofthis sampleofsubjectsinthetwoclinicaltrialsincludedinthis meta-analysis.WethereforesuggesttheconductionofRCTs involvingpatientswithAPSandprevalenceofarterial throm-bosis.
Funding
JFCarvalhoreceivedgrantsfromConselhoNacionalde Desen-volvimentoCientíficoeTecnológico–CNPQ(300665/2009-1) andfromFedericoFoundation.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.KeelingD,MackieI,MooreGW,GreerIA,GreavesM. Guidelinesontheinvestigationandmanagementof antiphospholipidsyndrome.BrJHaematol.2012;157: 47–58.
2.SangleNA,SmockKJ.AntiphospholipidAntibodySyndrome. ArchPatholLabMed.2011;135:1092–6.
3.SantamariaJR,BadziakD,BarrosMF,MandelliFL,CavalinLC, SatoMS.Síndromeantifosfolípide.AnBrasDermatol. 2005;80:225–39.
4.HanlyJG.Antiphospholipidsyndrome:anoverview.CMAJ. 2003;168:1675–82.
5.EspinosaG,CerveraR.Antiphospholipidsyndrome.Arthritis ResTher.2008;10:230.
6.HughesGRV.Antiphospholipidsyndrome(Hughes syndrome):10clinicaltopics.Lupus.2010;19:343–6.
7.PetriM.Managementofthrombosisinantiphospholipid antibodysyndrome.RheumDisClinNorthAmerica. 2001;27:633–42.
8.Lourenc¸oDM,LopesLH,VignalCV,MorelliVM.Avaliac¸ão clínicaelaboratorialdepacientesemusodeanticoagulantes orais.ArqBrasCardiol.1997;68:353–6.
9.CrowtherM,CrowtherMA.Intensityofwarfarincoagulation intheantiphospholipidsyndrome.CurrRheumatolRep. 2010;12:64–9.
10.Ruiz-IrastorzaG,HuntBJ,KhamashitaMA.Asystematic reviewofsecundarythromboprophylaxisinpatientswiththe antiphospholipidantibodies.ArthritisRheum.
2007;57:1487–95.
11.DelPapaN,VasoN.Managementofantiphospholipid syndrome.TherAdvMusculoskelDis.2010;2:221–7.
12.TuthillJI,KhamashtaMA.Managementofantiphospholipid syndrome.JAutoimmun.2009;33:92–8.
13.KhamashtaMA,CuadradoMJ,MujicF,TaubNA,HuntBJ, HughesGR.Themanagementofthrombosisinthe antiphospholipid-antibodysyndrome.NEnglJMed. 1995;332:993–7.
14.RosoveMH,BrewerPM.Antiphospholipidthrombosis:clinical courseafterthefirstthromboticeventin70patients.Ann InternMed.1992;117:303–8.
15.Ruiz-IrastorzaG,KhamashtaMA,HuntBJ,EscuderoA, CuadradoMJ,HughesGR.Bleedingandrecurrentthrombosis indefiniteantiphospholipidsyndrome:analysisofaseriesof 66patientstreatedwithoralanticoagulationtoatarget internationalnormalizedratioof3.5.ArchInternMed. 2002;162:1164–9.
16.DerksenRHWM,GrootPhG,KaterL,NieuwenhuisHK. Patientswithantiphospholipidantibodiesandvenous thrombosisshouldreceivelongtermanticoagulant treatment.AnnRheumDis.1993;52:689–92.
17.Krnic-BarrieS,O’ConnorCR,LooneySW,PierangeliSS,Harris EN.Aretrospectivereviewof61patientswith
antiphospholipidsyndrome.Analysisoffactorsinfluencing recurrentthrombosis.ArchInternMed.1997;157:2101–8.
18.Mu ˜noz-RodriguezFJ,FontJ,CerveraR,ReverterJC,TàssiesD, EspinosaG,etal.Clinicalstudyandfollow-upof100patients withtheantiphosphlipidsyndrome.SeminArthritisRheum. 1999;29:182–90.
19.WittkowskyAK,DowningJ,BlackburnJ,NutescuE.
antibodysyndromemanagedinananticoagulationclinic. ThrombHaemost.2006;96:137–41.
20.LimW,CrowtherMA,EikelboomJW.Managementof antiphospholipidantibodysyndrome:asystematicreview. JAMA.2006;295:1050–7.
21.LevineMN,RaskobG,BeythRJ,KearonC,SchulmanS. Hemorrhagiccomplicationsofanticoagulanttreatment:the seventhACCPconferenceonantithromboticand
thrombolytictherapy.Chest.2004;126:287S–310S.
22.WilsonWA,GharaviAE,KoikeT,LockshinMD,BranchDW, PietteJC,etal.Internationalconsensusstatementon preliminaryclassificationcriteriafordefinite
antiphospholipidsyndrome:reportofaninternational workshop.ArthritisRheum.1999;42:1309–11.
23.MiyakisS,LockshinMD,AtsumiT,BranchDC,BreyRL, CerveraR,etal.Internationalconsensusstatementonan updateoftheclassificationcriteriafordefinite
antiphospholipidsyndrome(APS).JThrombHaemost. 2006;4:295–306.
24.JadadAR,MooreRA,CarrollD,JenkinsonC,ReynoldsDJ, GavaghanDJ,etal.Assessingthequalityofreportsof randomizedclinicaltrials:isblindingnecessary?ControlClin Trials.1996;17:1–12.
25.DownsSH,BlackN.Thefeasibilityofcreatingachecklistfor theassessmentofthemethodologicalqualitybothof randomizedandnon-randomizedstudiesofhealthcare interventions.JEpidemiolCommunityHealth.1998;52: 377–84.
26.ReviewManager(RevMan)[Computerprogram].Version5.2. Copenhagen:TheNordicCochraneCentre,TheCochrane Collaboration,2012.
27.MantelN,HaenszelMW.Statisticalaspectsoftheeanalysisof datafromretrospectivestudiesofdisease.JNatCancerInst. 1959;22:719–48.
28.DeeksJJ,HigginsJPT,AltmanDG.Chapter9:Analysingdata andundertakingmeta-analyses.In:HigginsJPT,GreenS,
editores.CochraneHandbookforSystematicReviewsof Interventions.Version5.0.1[updatedSeptember2008].The CochraneCollaboration,2008.Disponívelem:
<http//www.cochrane-handbook.org>.
29.MannocciA.TheMantel-Haenszelprocedure.50yearsofthe statisticalmethodforconfounderscontrol.ItalJPublic Health.2009;6:338–40.
30.CrowtherMA,GinsbergJS,JulianJ,DenburgJ,HirshJ, DouketisJ,etal.Acomparisonoftwointensitiesofwarfarin forthepreventionofrecurrentthrombosisinpatientswith theantiphospholipidantibodysyndrome.NEnglJMed. 2003;349:1133–8.
31.FinazziG,MarchioliR,BrancaccioV,SchincoP,WisloffF, MusialJ,etal.Arandomizedclinicaltrialofhighintensity warfarinvs.conventionalantithrombotictherapyforthe preventionofrecurrentthrombosisinpatientswiththe antiphospholipidsyndrome(WAPS).JThrombHaemost. 2005;3:848–53.
32.Girón-GonzálezJA,DelRíoEG,RodríguezC,
Rodríguez-MartorellJ,SerranoA.Antiphospholipidsyndrome andasymptomaticcarriersofantiphospholipidantibody: prospectiveanalysisof404individuals.JRheumatol. 2004;31:1560–7.
33.AmesPRJ,CiampaA,MargaglioneM,ScennaG,IannacconeL, BrancaccioV.Bleedingandre-thrombosisinprimary antiphospholipidsyndromeonoralanticoagulation.Thromb Haemost.2005;93:694–9.
34.RieraR,AbreuMM,CiconelliRM.Revisõessistemáticase meta-análisesnareumatologia.RevBrasReumatol. 2006;46:8–11.
35.AtallahAN.Revisõessistemáticasdaliteraturae meta-análise.DiagnTratamento.1997;2:12–5.