www.jped.com.br
ORIGINAL
ARTICLE
Comparison
of
biochemical
and
immunological
profile
of
pediatric
patients
with
acute
myeloid
leukemia
in
relation
to
healthy
individuals
夽
,
夽夽
Fabiane
L.F.Z.
Sanches
a,∗,
Taís
M.
Nitsch
b,
Maria
Marluce
S.
Vilela
b,
Valdemiro
C.
Sgarbieri
caNutritionCourse,CenterforBiologicalandHealthSciences,UniversidadeFederaldeMatoGrossodoSul,CampoGrande,
MS,Brazil
bCentreforResearchinPediatrics,DepartmentofPediatrics,FacultyofMedicalSciences,UniversidadeEstadualdeCampinas
(UNICAMP),Campinas,SP,Brazil
cDepartmentofFoodandNutrition,FacultyofFoodEngineering,UniversidadeEstadualdeCampinas(UNICAMP),Campinas,
SP,Brazil
Received15September2014;accepted2December2014 Availableonline26June2015
KEYWORDS
Pediatrics; Leukemia; Biochemical assessment;
Immuneevaluation
Abstract
Objective: Tocomparethebiochemicalandimmunologicalprofilesofpediatricpatientswith
acutemyeloidleukemia(AML)withhealthychildrenandadolescents.
Methods: Thiswasacross-sectionalstudyinwhich21therapy-naïvepatientswithAMLwere
compared withagroupof24 healthyindividuals. The followingdata wereanalyzed: serum proteins,leucocytesandsubgroups,erythrocytes,hematocrit,hemoglobin,platelets,cytokines inperipheralbloodmononuclearcellsculturesunderspontaneousandBCG-orPHA-stimulated conditions,immunoglobulinA,anderythrocyticglutathione.Statisticalanalysiswasperformed usingSPSSsoftware,consideringassignificantp-values<0.05.
Results: Serumalbuminlevelswerehigher(p<0.0001)inthecontrolgroup,aswellasallthe
parametersrelatedtoredbloodcells(p<0.0001).Forleucocytesandsubgroups,nostatistical differencewasfoundbetweentheAMLandthecontrolgroups.Forcytokines,theconcentrations weresignificantlyhigherunderspontaneousandBCG-stimulatedconditionsforTNF-␣,IL-6, IL-10,andIFN-␥inthecontrolgroup.UnderPHA-stimulatedconditions,theconcentrationwas higher(p=0.002)onlyforIL-6.Nodifferencewasfoundbetweenthetwogroupsfortheother cytokines andfor IgA inthe saliva.Erythrocytic glutathione was higher(p<0.0001)inAML patients.
夽 Pleasecitethisarticleas:SanchesFL,NitschTM,VilelaMM,SgarbieriVC.Comparisonofbiochemicaland immunologicalprofileof
pediatricpatientswithacutemyeloidleukemiainrelationtohealthyindividuals.JPediatr(RioJ).2015;91:478---84.
夽夽
StudyconductedatUniversidadeEstadualdeCampinas(UNICAMP),Campinas,SP,Brazil.
∗Correspondingauthor.
E-mail:fabianelaflor@gmail.com(F.L.F.Z.Sanches). http://dx.doi.org/10.1016/j.jped.2014.12.004
Conclusions: Itwaspossibletocharacterizethebiochemicalandimmunologicalprofileof pedi-atricpatientswithAML,aswellashighlightsomesignificantdifferencesintheseparameters whencomparingwithhealthychildrenandadolescents.
©2015SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
PALAVRAS-CHAVE
Pediatria;
LeucemiaMieloide
Aguda;
Avaliac¸ãoBioquímica;
Avaliac¸ão
Imunológica
Comparac¸ãodoperfilbioquímicoeimunológicodepacientespediátricoscom leucemiamieloideagudaedeindivíduossaudáveis
Resumo
Objetivo: Compararoperfilbioquímicoeimunológicodepacientespediátricosportadoresde
LeucemiaMieloideAguda(LMA)emrelac¸ãoaumgrupodecrianc¸aseadolescentessaudáveis.
Métodos: Estudotransversal, emqueforamavaliados21pacientescomLMAvirgensde
ter-apiaeumgrupode24indivíduossaudáveis.Foramanalisadas:proteínasséricas,leucócitose subgrupos,eritrócitos,hematócrito,hemoglobinaeplaquetas,citocinasemculturadecélulas mononuclearesdosangueperiféricosobcondic¸ãoespontâneaeestimuladaporBCGouPHA, imunoglobulinaAeglutationaeritrocitária.Análiseestatísticafoirealizadaatravésdosoftware SPSSconsiderandop<0,05.
Resultados: Albuminaséricafoisuperior(p<0,0001)nogrupodecontrole,bemcomo,todos
osparâmetrosrelacionadoscomosglóbulosvermelhos(p<0,0001).Paraosleucócitose sub-grupos não houve diferenc¸aestatística entre ospacientescom LMAe ogrupo controle. As concentrac¸õesforamsignificativamentemaiselevadassobcondic¸õesespontâneaeestimulada porBCGparaascitocinasTNF-␣,IL-6,IL-10eIFN-␥nogrupocontrole.Sobcondic¸ãoestimulada comPHAaconcentrac¸ãofoisuperior(p=0,002)apenasparaaIL-6.Nãohouvediferenc¸a estatís-ticaparaasdemaiscitocinaseparaIgAsalivarentreosdoisgrupos.Glutationaeritrocitáriafoi superior(p<0,0001)nospacientesLMA.
Conclusões: Diantedoexpostofoipossívelcaracterizaroperfilbioquímicoeimunológicode
pacientes pediátricos com LMA, bem como, evidenciar diferenc¸as significativas em alguns dessesparâmetrosaosecompararosindivíduosdoenteseogrupodecrianc¸aseadolescentes saudáveis.
©2015SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos reservados.
Introduction
Commontypesofcancerinthepediatricagerangeinclude
leukemias, particularly acute lymphocytic leukemia (ALL)
andacutemyeloidleukemia(AML),centralnervoussystem
tumor, lymphoma,neuroblastoma, Wilmstumor,
osteosar-coma,andEwing’ssarcoma.Childrencomprise80%ofALL
cases,andonly10%ofAMLcases.Anincreasingprevalence
ofpediatriccancerhasbeenobservedinBrazil.1---3
Acute leukemia is a primary neoplasia of the bone medulla, characterized by a heterogeneous group of dis-easesinwhichthereisasubstitutionofnormalmedullary andbloodelementsbyimmaturecells(blasts)and accumu-lationofthesecellsinothertissues.4
According toCasciato,5 leukemic cells replicateslower
than the corresponding normal cells. Hematopoiesis is abnormal even before the proportion of cells in the medulla shows a perceptible increase. The precursors of immature leucocytes exhibiting malfunctioning progres-sively substitute the bone medulla and infiltrate other tissues.
Thesignsandsymptomsofacuteleukemiaresultfromthe dropofbloodcells,whichleadtoanemia,weakness,general discomfort,paleness,fatigue,palpitation,anddyspneato
exercise.Feverandinfectionsmayoccurasaconsequence ofdecreasedgranulocytes.5,6
The characterization of the biochemical and immuno-logicalprofile of AMLpatients is importantfor nutritional and medical interventions and following normal growth and development of children and adolescents, in order to improve the immunological response and tolerance of patientstotreatment,aswellastheirqualityoflife.
This study aimed to compare the biochemical and immunological profiles of pediatric patients with AML to thoseofhealthyindividualsmatchedinage.
Methods
Population
Thestudiedsamplecomprisedpatientsadmittedtothe
Cen-troInfantilBoldrini, Campinas-SP,Brazil, fortreatment of
leukemia,immediatelyafterthediagnosticofAML,and
par-ticipationofaclinicaltrialofnutritionalintervention.
The inclusion criteriawere:a confirmed diagnostic for
AML;treatment naivety; age range0---19 years;having an
guardian,afterbeinginformedoftheobjectivesand
meth-ods employed in the research and being aware of the
proceduresanddiscomfortstowhichparticipantswouldbe
submitted,beingfreetoaccepttoparticipateornotwithout
constraint.
Thestudyalsoincludedacontrolgroupofhealthy
chil-drenandadolescentsinthesameagerange.Theinclusion
criteriaforthisgroupwereindividualswithoutany
pathol-ogynorreceivinganymedicationatthetimeofselectionor
samplecollectionwhosignedaninformedconsent.
Attheendoftheinvestigation,21patientswithAMLhad
beenevaluated:11femalesand10males,withmedianage
6.83years(0.58---19.83).Thecontrolgroupwascomposedof
24individuals amongchildren andadolescentsdistributed
between 17 females and seven males, median age 9.67
(1.5---18.25) years.The age of the groups wasnot
signifi-cantlydifferent(p=0.351)accordingtotheMann---Whitney
test.
Biochemicalandimmunologicalevaluation
Samplecollection
Thebiologicalsampleswerecollectedfromthepatientsby
the nursing team of the Instituto InfantilBoldrini, where
allpatientswerediagnosedandrecruited.Between15and
20mLofbloodwerecollected,carefullyavoidinghemolysis.
Bloodfromhealthyindividualswascollectedinthe
out-patientclinic of the Centro de Investigac¸ão em Pediatria
(CIPED)bythenurseincharge.Forsalivacollection,a
plas-tic 1mL sterile and rejectable Pasteur pipette was used.
The participants wereinstructed not toeat or drink
any-thingexceptwater1hbeforecollectionandtopracticeoral
hygienebywashingthemouthwithpurewater.
Dosagesofbloodserumproteins
Serumprealbumin fractionwas determinedby
nephelom-etryand the serumalbumin by the colorimetric reaction
withbromocresolgreenusingspectrophotometry.The
anal-yseswereperformedinthebiochemistrylaboratoryofthe
DepartmentofClinicalPathology(DCP),oftheHospitaldas
Clínicas of the Universidade Estadual de Campinas
(UNI-CAMP).
Hemograms
The hemograms were performed at the Instituto Infantil
Boldriniaspartofa routinein theclinicaltreatment. For
healthy individuals (control group), the hemograms were
performed at the Laboratory of Clinical Pathology of the
HospitaldasClínicas(UNICAMP).
Reducederythrocyticglutathione
The assay followed a minor modification of the method
describedbyBeutler,7proposedbyPenna8200Lof
periph-eralblood EDTAwerelysedwith1.8mLofdistilledwater. Then, 2mL of 1.67% metaphosphoric acid solution were addedandthemixturewasfiltered.4mLof0.3MNa2HPO4
solutionwereaddedto1mLoftheclearfiltrateandreadat 412nmonaBechmanspectrophotometer.Asecondoptical densityreading wastakenafterthe additionof100mLof dithiobisnitrobenzoicacid(DTNB) solutiontothefiltrates. Resultswereexpressedinmg/dL.
Stimuliusedincultures
LyophilizedBCG(MoreauRiodeJaneirovaccinevials)were
freshly reconstituted with a culture medium RPMI 1640
(Sigma---Aldrich, USA) and used at 5×105UFC/mL.
Phy-tohemagglutinin (PHA, Sigma---Aldrich, USA) was used a
non-specific positive control at 7.5g/mL, and medium
alonewasusedasanegativecontrol.
Cytokineconcentrationinculturesupernatantsof peripheralbloodmononuclearcells(PBMC)
CytokineconcentrationwasmeasuredinPBMCcultures,by
amodificationoftheprotocolofGainesetal.9FreshPBMC
frompatientsandcontrolswereisolatedbydensity gradi-entcentrifugationoverHystopaque®(Sigma---Aldrich,USA),
washed, dilutedto 2×106cells/mL in RPMI1640 medium (Sigma---Aldrich, USA) supplemented with 10% human AB serum(Sigma---Aldrich,USA),1%glutamine(Sigma---Aldrich, USA), and 0.1% gentamycin and stimulated for 48h with reconstitutedBCG,PHA,ormediumaloneat37◦Cwith5% CO2inround-bottomed96-welltissuecultureplates(Nunc,
ThermoFisherCientific,USA).
Culturesupernatantswerecollectedandstoredat−80◦C for two-monoclonal antibody sandwich enzyme linked immunosorbent assay(ELISA).Commercialkits (DuosetRD Systems,UnitedStates)wereusedtomeasureinterferon-␥
(IFN-␥),tumornecrosisfactor-␣(TNF-␣),interleukin-6 (IL-6),andIL-10concentration,accordingtothemanufacturer’s protocol.Thelevelsofthetransforminggrowthfactorbeta (TGF-),andIL-8weremeasuredinbloodplasma.All sam-ples were measured in duplicates and the results were expressedinpg/mL.
ImmunoglobulinAinsaliva
The collectedsaliva wascentrifugedat 1000×gfor 7min
and then stored at −80◦C until time for analysis; the
results were expressed in mg/mL. The concentration of
immunoglobulinA(IgA)wasperformedbynephelometry.
Ethicalaspects
All ethical aspects were observed as recommended for
biomedical researchinvolving human beings,according to
theNationalHealthCouncilresolutionNo.196of1996.The
research protocol was approved by the National
Commit-teeforEthicsinResearch(CONEP)andregisteredunderNo.
14097.
Statisticalanalysis
All results were analyzed using SPSS® for Windows (Inc.
Released 2007. SPSS for Windows, version 16.0, USA).
Descriptive analysis of the variables were presented as
median (minimum andmaximum values).The comparison
ofbiochemicalevaluationamongpatientsandcontrolgroup
wasperformedusingtheMann---Whitneynon-parametrictest
atp<0.05.
Results
The present study included 21 patients with AML and 24
healthy subjects paired in age, with no statistical
200
150
100
50
0
AML Control
Erythrocytic glutathione
(mg/dL)
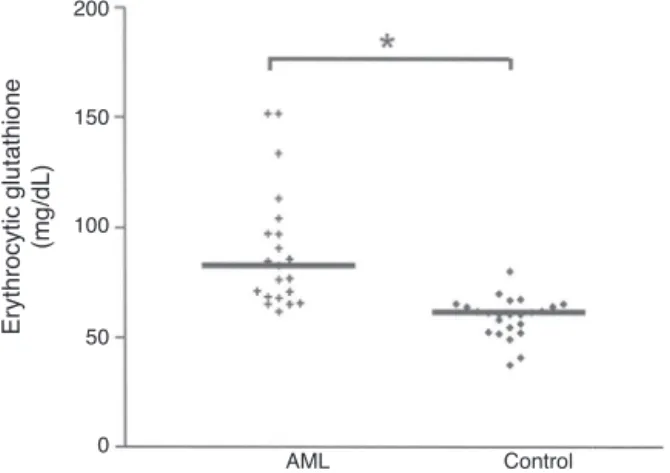
Figure 1 Erythrocytic reduced glutathione concentration (mg/dL)ofacutemyeloidleukemiapatientsandhealthy sub-jects(control)inthesame agerange.*Significantdifference: p<0.05,calculatedbytheMann---WhitneyTest.
Using the French American British (FAB) classification,
nine patients were classified as M5; four, as M2; one, as
M1;andone,asM7.Sixpatientsdidnothavethesub-type
specifiedintheirmedicalrecords.
Albuminandprealbuminserumproteins
Table1presentsthecomparativeresultsofserumalbumin
andprealbuminlevelsforAMLpatientsandcontrols.Itwas observedthatbothserumalbuminandprealbuminwere sig-nificantlyhigher(p<0.0001)inthehealthyindividualswhen comparedwiththeAMLpatients.
Hematologicalparameters
Table 2 presents the comparative results for the
hema-tological parameters between AML patients and healthy individuals.
Therewasnostatisticaldifferenceinthemedianlevels oftotalleucocytesandsubgroups(lymphocytes,monocytes and granulocytes) between the twogroups. However, the highervaluesoferythrocytes,hematocrit,hemoglobinand platelets(p<0.0001) inthecontrolgroup whencompared withtheAMLpatientsarenoteworthy.
Erythrocyticreducedglutathione(GSH) concentration
The median GSH concentration in theerythrocyte of AML
patientswassignificantlyhigher(p<0.0001)thanthevalues
measuredinhealthysubjects,asillustratedinFig.1.GSH
concentrationwas82.88(61.94---151.77)mg/dLintheAML group,comparedto61.72mg/dLinthecontrolgroup.
CytokineconcentrationinsupernatantsofPBMC cultures
Comparative production of cytokines was determined in
supernatantsofperipheralbloodmononuclearcellsculture
(PBMC) under three different conditions for the AML
and control group: spontaneous (culture medium without
stimulation); culture medium plus the vaccine BCG; and
culturemediumplusphytohemagglutinin(PHA).Theresults,
expressedasmediumvaluesandthespread(min-max),are
showninTable3.
Underspontaneouscondition,TNF-␣ andIFN-␥ concen-trationsweresignificantlyhigherinthe AMLpatientsthan inhealthysubjects.Nodifferencewasfoundbetweenthe twogroupsforIL-6andIL-10,underthiscondition.
Under BCG-stimulated condition, all cytokines concen-trations(TNF-␣,IL-6,IL10,IFN-␥)weresignificantlyhigher thaninthecontrolgroup.
InthepresenceofPHA,onlyIL-6concentrationwas sig-nificantlyhigher(p<0.002)inthecontrol,whencompared withtheAMLgroup.TNF-␣wasalsohigherthecontrolthan intheAMLgroup;however,itdidnotreachstatistical sig-nificance.Nodifferencewasfoundbetweenthetwogroups forIL-10andIFN-␥underthiscondition.
The concentration of TGF- and IL-8, which was mea-sured in the blood plasma in both groups, and only in the presence of BCG, did not show statistical difference betweenthegroups(datanotshown).
DeterminationofIgAinsaliva
ImmunoglobulinA(IgA)wasdeterminedinthesalivaofthe
subjectsinbothgroupsofstudy.
TheconcentrationofIgAwas6.5(1---17.4)mg/dLforthe
AMLgroupand9.05(1.8---25.1)mg/dLforthecontrolgroup,
showingnodifference(p=0.693)betweenthegroups.
Discussion
Serumproteinshavebeenusedasasensitiveindicatorfor
undernutrition.However,otherfactorsnotrelatedto
nutri-tionalconditionmayaffectthelevelofserumprotein,such
asstateofhydration,reductionofhepaticproteinsynthesis,
alteredcapillarypermeabilityduetoinfectionsorzinc
defi-ciencycatabolisminperiodsofstress,andhypermetabolism
incertainneoplasias.10,11
The present albumin (4.8g/dL) and prealbumin (21.45mg/dL) results in the control group were statis-ticallyhigher(p<0.0001)thanthevaluesfoundintheAML patients.Theseresultsreflectdifferencesexpectedinthe nutritionalstateofhealthychildrenandadolescentswhen comparedwithage-matchedAMLpatients.
Inastudyperformedon226adultpatientswithcancer, MarínCaroetal.12 found that 32%of the studied
popula-tionpresented serumalbumin concentration between 3.0 and3.5g/dL(mild undernutrition),demonstratinga nega-tivecorrelationbetweenserumproteinlevelsandfeeding problem.
Severely ill patients normally present metabolic alter-ations in carbohydrates, lipids, and proteins. Such alter-ations result from increased need of energy and protein catabolism, contributing to immunological and gastroin-testinalalterations.13
Table1 Levelsofserumalbuminandprealbumininacutemyeloidleukemia(AML)patientsandinhealthyindividuals(control) inthesameagerange.
Serumprotein AML
(n=21)
Control (n=24)
Albumin Mediana 3.6 4.8
min---max (2.6---4.6) (4.4---5.4)
pb <0.0001
Prealbumin Mediana 11.75 21.45
min---max (3.97---24.0) (13.1---34.6)
pb <0.0001
aMedianofminimumandmaximum.
b p-valuecalculatedbytheMann---Whitneytest.
Table2 Hematologicalparametersdeterminedinpediatricacutemyeloidleukemia(AML)patientsandinhealthyindividuals (control)inthesameagerange.
Parameters AML(n=21) Control(n=24) pa
Leucocytes(cell×109/L) 13.1b(1.1---494.0) 6.51b(1.79---15.11) 0.219
Lymphocytes(cell×109/L) 2.3(0.6---58.4) 2.8(1.83---8.64) 0.368
Monocytes(cell×109/L) 1.3(0.1---10.6) 0.45(0.24---0.91) 0.308
Granulocytes(cell×109/L) 4.3(0.1---48.6) 3.64(1.91---5.57) 0.825
Erythrocytes(cell×1012/L) 2.55(1.78---4.36) 4.82(4.17---5.4) <0.0001
Hematocrit(%) 23.4(16.6---36.6) 40.7(34.1---47.2) <0.0001 Hemoglobin(g/dL) 7.7(5.6---12.5) 13.55(11.1---15.5) <0.0001 Plalelets(cell×109/L) 38.0(16---220) 307.0(162---461) <0.0001
ap-valuecalculatedbyMann---Whitneytest. b Medianofminimumandmaximum.
andgranulocytes,althoughthemedianvaluestendedtobe
higherintheAMLgroup.Thislackofstatisticalsignificance
mightbeexplainedasafunctionoftwofactors:(a)the
rel-ativelysmallnumberofsubjectsinthepresentsamples;(b)
thegreat variability normallyfound for white blood cells
(leucocytes)inpatientswithleukemia.5
Vianaetal.14conductedastudyon83childrenwithAML
and also found a wide variation in the number of leuco-cytes/mLofblood.
In the present study, the total number of leucocytes (13.1×109cells/L) for the AML group can be considered slightly above normal and was normal for the control
Table3 Cytokinesconcentration(pg/mL)determinedinsupernatantsofperipheralbloodmononuclearcellsculturesofacute myeloidleukemia(AML)patientsandhealthysubjects(control)inthesameagerange.
Cytokines Group Conditions
Spontaneous BCG PHA
TNF-␣ AML 40.491a(0.0---418.9) 831.47a(41.01---15,825.3) 683.17a(38.6---1977.2)
Control 22.42(0.0---316.7) 4800.42(1408.7---13,101.1) 986.61(87.7---27,882.1)
pb 0.011 <0.0001 0.052
IL-6 AML 455.641(0.0---10,328.2) 4807.2(41.0---31,186.7) 3010.4(54.2---28,250) Control 736.17(19.8---5044.4) 28,961.67(25,176.7---293,344.6) 9654.65(1904---29,051.1)
pb 0.197 <0.0001 0.002
IL-10 AML 0.0(0.0---1796.8) 124.54(0.0---1783.05) 393.80(63.8---1127.9) Control 0.0(0.0---119.7) 615.46(118.6---2204.9) 627.52(61.5---1465.2)
pb 0.065 0.003 0.284
IFN-␥ AML 23.17(0.0---70.6) 98.71(0.0---1665) 150.23(0.0---3913.8) Control 0.0(0.0---48.9) 676.77(0.0---1935.3) 87.96(0.0---630.5)
pb 0.012 0.003 0.273
TNF,tumornecrosisfactor;IL,interleukin;IFN,interferon.
(6.5×109cells/L),sincethereferencevaluesrangefrom4.5
to10.5×109cells/L.
Conversely, erythrocytes,hematocrit, hemoglobin, and
plateletswereallsignificantlyhigher(p<0.0001)inthe
con-trol groupwhen compared withthe AML group (Table 2).
Thesedataappeartoexplainthestrongtendencyforanemia inAMLpatients.
Thecell-mediatedimmunityagainsttumorscanincrease by expression of cytokines and co-stimulators in tumoral cellsandbytreatment oftumorscarryingindividualswith cytokines,whichstimulatetheproliferationand differenti-ationofTlymphocytesandnatural killer(NK)cells. IFN-␥
and TNF-␣ are considered efficient antitumoral agents in animal models, but their use in patients is limited due to serious toxic collateral effects. Growth factors, such as granulocytes---macrophages colonies stimulating factor (GM-CSF) andgranulocytes colonies stimulating factor (G-CSF) are usedin cancer treatment protocols to decrease the periods of neutropenia and thrombocytopenia after chemotherapyorbonemedullaautologoustransplant.15
One study compared the levels of pro-inflammatory cytokines in adult patients with cancer with a group of healthysubjects(control).Itwasfoundthatthebloodserum levelsofIL-1andTNF-␣werehigher(p<0.0001)inpatients withcancer,whencomparedwiththecontrolgroup.16
In the present study, the spontaneous production of TNF-␣ andIFN-␥insupernatantsofPBMCwassignificantly higherinAMLpatients,suggestingthatoxidativestressand inflammationareinvolvedinAML.Conversely,therewasno significantdifferencebetweengroupsinproductionofIL-6 andIL-10,whichactasregulatorycytokines.
There is increasing evidence in the literature that the ROS-inducedgenerationof oxidative stress playsarolein leukemia.Astrongassociationbetweenoxidativestressand theincidenceofdiseaserelapsewasdemonstratedbyZhou et al.17 These investigators showed that the activities of
adenosinedeaminase andxanthineoxidasewerehigherin AMLrelapsecondition,whereasthoseofglutathione perox-idase,monoamineoxidase,superoxidedismutase, andthe total antioxidantcapacity (T-AOC) were lower in the pri-maryconditionthanincontrols.Accordingtotheseauthors, oxidative stress is a crucial feature of AML and probably affectsitsdevelopmentandrelapses.
Inthepresentstudy(Table3),allcytokinesinvestigated showed significantly higher concentrations in the control groupwhencomparedwiththeAMLgroupinthepresence ofBCGvaccine,aspecificantigenicfactor, whichsuggests alowercapacityofAMLpatientstopositivelyreactto anti-gens. Conversely, in the presence of PHA, a non-specific mitogen stimulator, only IL-6 showed significantly higher concentrationinthecontrol(p<0.002)whencomparedwith theAMLgroup.TNF-␣,IL-10,andIFN-␥didnotshow differ-encebetweenthetwogroups.
Adequate concentrationof glutathioneis necessary for normalcellproliferationincludinglymphocytesand epithe-lial cells in the intestine.18 Glutathione (GSH) is also
essential for the activation of T lymphocytes and poly-morphonuclear leucocytes and also for the production of cytokines;therefore it is indispensable for theexpression of the immune response in situations of immunological challenges.19 Theauthors citedabovementionedthat the
cellular concentrations of GSH are drastically reduced in situations such as protein undernutrition and oxida-tive stress, and in various pathological conditions, such asprotein-energy undernutrition, acquired immunological deficiency(AIDS),andadvancedneoplasias.
Astudy by Russo etal.20 demonstratedthat the levels
ofGSHincancer cellsmaybeseveralfoldshigherthanin correspondingnormal cells. AccordingtoEngin,21 the
lev-elsoferythrocyticglutathionewere31%higherinpatients withlocalized carcinoma and 78% higher in patients with metastaticcancer comparedwithhealthy controls. These resultsmayreflectthehighlevelsofGSHintumorsandalso thatthesehighlevelsmaybeassociatedwithresistanceof tumoralcellstochemotherapeutictreatment.
Theresultsofthepresentareinaccordancewiththoseof Russoetal.20 andEngin,21revealingahigherconcentration
ofGSHintheAMLpatients(p<0.0001)whencomparedwith thehealthysubjectgroup(control),asillustratedinFig.1. Recentpublishedstudy22indicatedthatCD34+AMLcells
haveelevated expression ofmultiple glutathionepathway regulatory proteins, presumably asa mechanism to com-pensate to increased oxidative stress in leukemic cells. ConsistentwiththisobservationCD34+AMLcellshavelower
levels of reduced (GSH) and increased levels of oxidized (GSSG)glutathione.Thesefindingssuggesttheintrinsic bal-anceandhomeostasisandtheGSHtoGSSGrationisaltered (aberrant)intheCD34+AMLcells.
The above cited authors propose that the decreased GSH level is due to higher consumption of GSH in sev-eralprocessesrequired for cancer cell survival including: (1)reductionofreactiveoxygenspecies,suchasH2O2;(2)
properS-glutathionylationof theproteome inresponse to oxidativestress;and(3)detoxificationofincreased produc-tionoflipidperoxides.
Thesesame authorsalsopresentednewagents suchas parthenolide(PTL)andpiperlongumine(PLM)havinga dra-maticinhibitoryeffectontheleukemicGSHsystem,whereas onlya limited andtransient perturbation in normal cells. The same group of researchers had previously shown,23
the PTL effectively eradicated AML stem and progenitor cellsthataretypicallyresistant/refractorytoconventional chemotherapy.
SecretorysalivaryimmunoglobulinA (sIgA)is an impor-tantparameterforevaluatingtheimmunologicalstatusof the gastrointestinal mucosa, with the advantage of using noninvasive procedure and essentially without discomfort tothepatient.Itisconsideredthemostimportanthumoral mediatorforthemucosaimmunity,assistinginavarietyof protectivemechanisms.Itpresentshigherresistanceto pro-teolyticdegradationthananyotherclassofimmunoglobulin and can be found in the entire digestive and respiratory tracts, impeding the absorption of a large quantity of antigens.24,25
AccordingtoSouzaetal.,26inpatientswithcancer,the
synthesis of antibodies may bedecreased or exacerbated depending on the immunological mechanisms involved in the proliferation of tumoral cells, determining an eleva-tionor reductionintheconcentrations ofimmunoglobulin fractions.
betweentheAMLpatientsandthehealthycontrols,inspite ofdetectinghigherlevelsinthepatients.
In the comparison of pediatric patients with AML with normal subjects matched for age some conclusions could bedrawn: a)the AML group presented significantlylower concentrationsofserumalbuminandprealbumin, suggest-ingimminentdangerofproteinundernutrition:b)theAML patientsshowedlowervalues(p<0.0001)forallredblood cells parameters, suggesting a state of anemia in these patients; (c) the statistic lower production of cytokines ofAML groupunderspontaneousandBCG-stimulated con-ditions appears to indicate a drop in the immunological responseofpatientscomparedtothenormalsubjects;(d) thesignificantly higher GSH concentrationin the erythro-cytesofAMLpatients,comparedtocontrols,mayreflectthe aberrantglutathionemetabolismandhomeostasisdescribed inthereference.22
Funding
ThisstudyreceivedfundingfromCNPq.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
We thank National ResearchCouncil (CNPq), for financial
supportandforgrantingascholarshiptothefirstauthor.
References
1.BrasilMinistériodaSaúdeInstitutoNacionaldeCâncer(INCA). Câncernacrianc¸aenoadolescentenoBrasil:dadosdos reg-istrosdebasepopulacionaledemortalidade.RiodeJaneiro: MinistériodaSaúde;2008.
2.BrasilMinistériodaSaúdeInstitutoNacionaldeCâncer(INCA). Consensonacionaldenutric¸ãooncológica.RiodeJaneiro:INCA; 2011.
3.SilvaFF,ZandonadeE,Zouain-FigueiredoGP.Analysisof child-hoodleukemiamortalitytrendsinBrazil,from1980to2010.J Pediatr(RioJ).2014;90:587---92.
4.Elman I, Pinto-e-Silva ME. Crianc¸as portadoras de leucemia linfóideaguda:análisedoslimiaresdedetecc¸ãodosgostos bási-cos.RevBrasCancerol.2007;53:297---303.
5.CasciatoDA.Manualdeoncologiaclínica.SãoPaulo:Tecmedd; 2008.
6.ChauffailleML,CamposMG.Leucemias.In:ForonesNM,Filho RJ,TadokoroH,FreireCA,editors.Guiasdemedicina ambula-torialehospitalar:oncologia.Barueri,SP:Manole;2005.
7.BeutlerE,editor.Redcellmetabolism.NewYork:Churchill Liv-ingstone;1986.
8.PennaSP. Níveisdeglutationa reduzida eatividadeda cata-lase,superóxido dismutaseeglicose-6-fosfato desidrogenase emindivíduosexpostos aovapordemercúrio. [Dissertation]. Campinas,SP:UniversidadeEstadualdeCampinas;1995.
9.GainesH,AnderssonL,BiberfeldG.Anewmethodformeasuring lymphoproliferationatthesingle-celllevelinwholeblood cul-turesbyflowcytometry.JImmunolMethods.1996;195:63---72.
10.BottoniA,OliveiraGP,FerriniMT,WaitzbergDL.Avaliac¸ão nutri-cional:exameslaboratoriais.In:WaitzbergDL,editor.Nutric¸ão oral,enteraleparenteralnapráticaclínica.3rded.SãoPaulo: Atheneu;2000.p.279---94.
11.VitoloMR. Nutric¸ão:da gestac¸ãoaoenvelhecimento.Rio de Janeiro:Rubio;2008.
12.Marín Caro MM, Gómez Candela C, Castillo Rabaneda R, etal. Nutritionalrisk evaluationand establishmentof nutri-tionalsupportinoncologypatientsaccordingtotheprotocol of the Spanish Nutrition and Cancer Group. Nutr Hosp. 2008;23:458---68.
13.GarófoloA.Diretrizesparaterapianutricionalemcrianc¸ascom cânceremsituac¸ãocrítica.RevNutr.2005;18:513---27.
14.VianaMB,CunhaKC, Ramos G,Murao M.Leucemiamielóide aguda na crianc¸a: experiência de 15 anos em uma única instituic¸ão.JPediatr(RioJ).2003;79:489---96.
15.AbbasAK,LichtmanAH,PillaiS.Immunologiacelulare molec-ular.6thed.RiodeJaneiro:Elsevier;2011.
16.Logan RM, Stringer AM, Bowen JM, et al. The role of pro-inflammatory cytokines in cancertreatment-induced ali-mentary tract mucositis: pathobiology, animal models and cytotoxicdrugs.CancerTreatRev.2007;33:448---60.
17.ZhouFL,ZhangWG,WeiYC,et al.Involvementofoxidative stressintherelapseofacutemyeloidleukemia.JBiolChem. 2010;285:15010---5.
18.AwTY.Cellularredox:amodulatorofintestinalepithelialcell proliferation.NewsPhysiolSci.2003;18:201---4.
19.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glu-tathione metabolism and its implicationsfor health. JNutr. 2004;134:489---92.
20.RussoA,DeGraffW,FriedmanN,MitchellJB.Selective modu-lationofglutathionelevelsinhumannormalversustumorcells andsubsequentdifferentialresponsetochemotherapydrugs. CancerRes.1986;46:2845---8.
21.EnginA.Differencesinbloodglutathionelevelsofpatientswith advancedorlocalizedcarcinoma.Tumori.1995;81:132---4.
22.PeiS,MinhajuddinM,CallahanKP,etal.Targetingaberrant glu-tathionemetabolism to eradicatehumanacute myelogenous leukemiacells.JBiolChem.2013;288:33542---58.
23.Guzman ML, Rossi RM, Karnischky L, et al. The sesquiter-penelactoneparthenolideinduces apoptosisofhumanacute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163---9.
24.BrandtzaegP.Molecularandcellularaspectsofthesecretory immunoglobulinsystem.APMIS.1995;103:1---19.
25.LammME,NedrudJG,KaetzelCS,MazanecMB.IgAandmucosal defense.APMIS.1995;103:241---6.