w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Distribution
of
phytoestrogenic
diarylheptanoids
and
sesquiterpenoids
components
in
Curcuma
comosa
rhizomes
and
its
related
species
Vichien
Keeratinijakal
a,b,∗,
Sumet
Kongkiatpaiboon
c,∗ aDepartmentofAgronomy,FacultyofAgriculture,KasetsartUniversity,Bangkok,ThailandbNationalCenterforAgriculturalBiotechnology,KasetsartUniversity,Bangkok,Thailand
cDrugDiscoveryandDevelopmentCenter,ThammasatUniversity(RangsitCampus),Pathumthani,Thailand
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received30August2016 Accepted16December2016 Availableonline21March2017
Keywords:
WanChakModLoog Phytochemicals
Phytoestrogen-producingherb Diarylheptanoids
Sesquiterpenoids
a
b
s
t
r
a
c
t
CurcumacomosaRoxb.,Zingiberaceae,aphytoestrogen-producingherbwithvernacularlynamed“Wan ChakModLoog”inThailand,hasbeentraditionallyusedfortreatmentofgynecologicdiseasesandsold asfoodsupplementinthemarket.However,similarrhizomesofitsrelatedspeciesmayleadtothe confusionintheusesofthisplant.Thisstudywasaimedtoinvestigatethephytochemicalconstituentsof differentCurcumaspp.thatusedas“WanChakModLoog”.Characteristicmajorcompoundswereisolated andidentified.Phytochemicalanalysisof45CurcumasamplesrepresentingCurcumasp.,C.latifolia,andC. comosawereanalyzedandcomparedwiththeirphylogeneticrelationshipinferredbyAmplifiedFragment LengthPolymorphismanalysis.PhytoestrogendiarylheptanoidswerefoundinallsamplesofC.comosa
whilesesquiterpenoidsincludinghepatoxiczederonewerefoundinC.latifoliaandCurcumasp.samples. ©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
CurcumacomosaRoxb.,anindigenousmedicinalherb
vernac-ularlynamed “Wan Chak Mod Loog”in Thailand,is recognized
asa phytoestrogen-producingplantbelongingtofamily
Zingib-eraceae(Phupatanapongetal.,2001;Soontornchainaksaengand
Jenjittikul, 2010). Its roots have been used for the treatment
of fluatalence and gynecologic diseases such as premenstrual
syndrome(PMS),abnormalmenstruationand uterinepain.This
plantisconsideredasanactiveconstituentinvarioustraditional
woman drug preparation in South EastAsian countries. It has
estrogenic-likeactivity(Piyachaturawat etal.,1995a,b)andhas
beenextensivelyusedamongmenopausalwomen(Winuthayanon
etal.,2009a).Variouspharmacologicalactivitieshavealsobeen reported,e.g.anti-lipidemic(Piyachaturawatetal.,1995c,1999a), choleretic(Suksamrarnet al., 1997), estrogenic (Winuthayanon etal.,2009a,b;Bhukkhaietal.,2012),uterotroic(Piyachaturawat etal.,1995a),growthsuppressingonmale reproductiveorgans (Piyachaturawatetal.,1998),malefertility(Piyachaturawatetal.,
∗ Correspondingauthors.
E-mails:agrvck@ku.ac.th(V.Keeratinijakal),sumetk@tu.ac.th (S.Kongkiatpaiboon).
1999b), plasma cholesterol reduction (Piyachaturawat et al., 1999a),anti-inflammatory(Jantaratnotaietal.,2006;Sodsaietal., 2007), and anti-oxidant effect (Niumsakul et al., 2007). More-over,inovariectomizedratswithestrogendeficiency,thisplant
could protect bone loss and accelerate human osteoblast
pro-liferation and differentiation (Tantikanlayaporn et al., 2013a,b; Weerachayaphorn et al., 2011), enhance vascular relaxation (Intapadetal.,2009,2012),preventneuronlossandimprove learn-ingandmemoryfunction(Suetal.,2010,2011,2012a,b).
Phytochemical investigationof C. comosa revealed the
pres-enceof sesquiterpenoids(Xu etal., 2008; Qu et al., 2009) and diarylheptanoids(Suksamrarnetal.,2008).Diarylheptanoidsare ofinterestsincetheyexertedestrogenic-likeactivity(Suksamrarn etal.,2008;Winuthayanonetal.,2009a,b).However,itsC.comosa relatedspecies(e.g.C.latifoliaandC.elata)availableinthemarket alsohassimilarshapeofrhizomesandmayleadtotheconfusionin theusesofthisplant.Theinconsistencyofrawmaterialshampered theusage,development,and scientificresearchof theseplants. Therefore,aspecialconsiderationisneededwhenpurchasingthe
samplesfromthemarket.
In general,sesquiterpenoids mayexerta varietyof
pharma-cologicalproperties. However,hexaneextract ofrhizomesfrom C.elatacontaininghighproportionofsesquiterpenezederone(5) causedliverenlargementandscatteredwhitefociovertheorgans
http://dx.doi.org/10.1016/j.bjp.2016.12.003
V.Keeratinijakal,S.Kongkiatpaiboon/RevistaBrasileiradeFarmacognosia27(2017)290–296 291
atautopsy(Pimkaewetal.,2013).Zederone(5)andwasfoundtobe hepatotoxicinmice.However,itwasalsoisolatedfromC.comosa (Quetal.,2009).Whilethereisaverylargevariationinrhizome
morphologyof“WanChakModLoog”incultivation,the
pheno-typicplasticityofvegetativepartsof“WanChakModLoog”can
leadtothewrongtaxonomicassignment(Soontornchainaksaeng
andJenjittikul,2010).Therefore,thisstudyutilizedAmplified
Frag-mentLengthPolymorphism(AFLP)markerstechniquetoexamine
Curcumasamples(Keeratinijakaletal.,2010)togetherwiththeir morphologicalcharacteristicsandinvestigatedthebioactive con-stituentsofdifferentCurcumaspeciesthatareusedas“WanChak
ModLoog”.
InthecourseofidentifyingthegeneticdiversityofC.comosa
and its related species, the samples were collected from
dif-ferent provenances of Thailand (Keeratinijakal et al., 2010).
Additional samples were collected and 118 accessions were
used to elucidate the phylogenetic relationships.
Correspond-ing plant materials were cultivated under field conditions in
the National Corn and Sorghum Research Center of Kasetsart
Universityin theeast ofThailand.Majorconstituentswere iso-latedandidentified.Afterbeingcultivatedinthesamecondition
and harvested inthesame age,thechemical constituents
phy-toestrogen diarylheptanoids and sesquiterpenoids of different
Curcumasamplewereanalyzed.Theresultscouldidentifythemost productiveplantwithhighcontentsofactivephytoestrogen
con-stituents withnon-toxic compounds.The present papershould
providea basis for furtherpharmaceutical developmentof this plant.
Materialsandmethods
Plantmaterials
Atotalof118accessionsofCurcumaspp.,locallycalled“Wan ChakModLoog”,wascollectedinourpreviousstudy(Keeratinijakal etal.,2010)togetherwithsomeadditionalcollectionsfrom culti-vatedsitesthroughoutThailand.Allsamplesweregrownatthe
National Corn and Sorghum Research Center of Kasetsart
Uni-versity,Pakchong, Nakhon-Ratchasima province,Thailand.AFLP
analysis was conducted in order to identify and elucidate the
phylogeneticrelationships.Thefollowing characteristicsin each accessionwereobserved:plantheight,shapeofmasterrhizome, insidecolorofmasterrhizome,presenceofsessiletubers,presence ofredpathalongthemidrib,inflorescenceandflower morphol-ogy as described in our previous paper (Keeratinijakal et al., 2010).
Chemicals
HPLCgradeacetonitrilewaspurchasedfromFisherScientific (UK).DeionizedwaterwaspurifiedbyWaterProPS(Labconco,MO, USA).Allreagentswereofanalyticalgradeifnotstatedotherwise.
Extractionandisolation
TherhizomesofCurcumasamplesweredriedinanovenat60◦C,
andpulverized.Eachsamplewasstoredinanairtightplastic con-tainerandkeptinthedryplaceuntilused.Thesuccessiveextraction wasdoneby Soxhletextractorusinghexane, ethylacetate, and ethanol,respectively.Eachextractwasfilteredandthesolventwas
evaporatedtodrynessusingrotaryevaporatorandhighvacuum
pump.Fractionationwasdoneoncolumnchromatographyusing
solventmixturesofhexaneandethylacetatewithincreasing polar-ity.EachfractionwasmonitoredwithTLC,combinedandfurther
purifiedwithcolumnchromatography.
Fresh rhizomesof mixed Curcuma sp.and C. latifolia(75kg) yielded6.5kgofdriedcoursepowder.Successiveextractionusing Soxhletextractoryieldedthehexane,ethylacetate,andethanol
extracts of 150.32, 210.75 and 375.69g, respectively. Hexane
extractwassubjectedtocolumnchromatography(Mercksilicagel 60No.107734,particlesize0.063–0.20mm)withsolventmixtures ofhexane–ethyl acetate(ratiofrom10:0to0:10)with increas-ing polarity, yielding seven fractions (CLH1–CLH7) of 2.35mg, 1.87g,25.98g,34.56g,12.85g,28.14g,and47.96g,respectively.
Ethyl acetate extract was subjected to column
chromatogra-phy withsolventmixtures of hexane–ethylacetate (ratio from
10:0 to 0:10), and ethyl acetate–methanol (ratio from 10:0 to
0:10),withincreasingpolarity,yieldingfivefractions(CLA1–CLA5) of 1.45, 15.68, 35.84, 49.70, and 67.73g, respectively. Ethanol
extract was subjectedto column chromatography with solvent
mixturesofhexane–ethylacetate(ratiofrom10:0to0:10),and ethyl acetate–methanol (ratiofrom10:0 to0:10)with increas-ing polarity, yielding six fractions (CLE1–CLE6) of 3.45, 26.78, 28.40, 32.50, 69.25, and 56.76g, respectively. Further
purifica-tion wasdone using column chromatography (Mercksilica gel
60 No. 107734, particle size 0.063–0.20mm). Fraction CLH-3
using hexane–ethyl acetate(95:5) as an eluent yielded
germa-crone (1) (20mg). Fraction CLH-4 using hexane–ethyl acetate (9:1) asaneluent yielded furanodienone(2)(35mg) and curz-erenone(3)(44mg).FractionCLH-4usinghexane–ethylacetate (85:15)asaneluentyieldedcurdione(4)(75mg).Fraction CLH-5usinghexane–ethylacetate(8:2)asaneluentyieldedzederone (5)(7.8mg).
FreshrhizomesofmixedC.comosa(10kg)yieldeddriedcourse
powder of 2kg. Successive extraction using Soxhlet extractor
yieldedthehexane,ethylacetate,andethanolextractsof95.30, 150.10and275g,respectively.Hexaneextractwassubjectedto
columnchromatography (Mercksilicagel60 No.107734,
parti-cle size0.063–0.20mm)withsolventmixtures ofhexane–ethyl
acetate(ratiofrom10:0to0:10)withincreasingpolarity, yield-ingfivefractions(CCH1–CCH5)of2.25mg,0.97g,30.15g,28.45g, and10.55g,respectively.Ethylacetateextractwassubjectedto
columnchromatography withsolventmixturesofhexane–ethyl
acetate(ratiofrom10:0to0:10),andethylacetate–methanol(ratio from10:0to0:10)withincreasingpolarity,yieldedfivefractions (CCA1–CCA5)of2.48,5.46,20.45,32.15,and15.38g,respectively. Ethanolextractwassubjectedtocolumnchromatographywith sol-ventmixturesofhexane–ethylacetate(ratiofrom10:0to0:10),and ethylacetate–methanol(ratiofrom10:0to0:10)withincreasing polarity,yieldingfourfractions(CCE1–CCE5)of4.45,27.16,29.56, and33.54g,respectively.Furtherpurificationwasconductedusing
columnchromatography (Mercksilicagel60 No.107729,
parti-clesizelessthan0.063mm).FractionCCH-4usinghexane–ethyl acetate(75:25)asaneluent,yielded 1,7-diphenyl-(6E)-6-hepten-3-ol(6)(30mg).FractionCCE-3usinghexane–ethylacetate(40:60) asaneluent,yielded1,7-diphenyl-(4E,6E)-4,6-heptadien-3-ol(7) (25mg).
NMRandMS:Each purecompoundwasdissolvedin 99.98%
CDCl3 orin99.8%methanol(ca.5mg in0.7ml)andtransferred
into5mmNMRsampletube(Promochem,Wesel,Germany).
Spec-tra wererecorded bytheBrukerTopspinsoftware ona Bruker
Avance 400 spectrometer (Bruker, Rheinstetten, Germany). In
methanol-d4 smallamountsofmethanol-d1 wereusedas inter-nalstandardfor1H(␦
1,7-diphenyl-(6E)-6-hepten-3-ol(6)(Suksamrarnetal.,2008),and 1,7-diphenyl-(4E,6E)-4,6-heptadien-3-ol (7) (Suksamrarn et al., 2008).
Determinationofphytochemicalconstituentscontentsin
Curcumasamples
HPLC was performed on an Agilent 1100 series equipped
withaChemstationsoftware,degasserG1322A,quaternarypump
G1322A,thermoautosamplerG1329/1330A,columnovenG1316A,
anddiodearraydetectorG1315A.Theseparationwascarriedout onaSorbaxreversed-phaseC-18column(250×4.6mmi.d.,5m). Mobilephasesystemwas(A)deionizedwaterand(B)acetonitrile. Gradientelutionwasusedbylinearincreasingof mobilephase compositionsfrom50%to90%BinAfor25minand90%BinAfor 5min.Thecolumnwasequilibratedwith50%BinAfor5minprior eachanalysis.Theflow-ratewassetat1ml/minatambient tem-perature.Injectionvolumewas10l.Thedetectionwasmonitored at260nm.
EachCurcumasamplewaspreparedfromthreerhizomes cul-tivatedinthesamefieldconditionandageatNationalCornand SorghumResearchCenter.Therhizomesweresliced,driedin60◦C
hot air oven for 20h, ground, and passed through a sieve (60
mesh).Eachsample wasaccurately weighed(10g) and
succes-sivelyextractedwithhexaneandethanol(200mleach)for6husing Soxhletextractor.Theextractwasfilteredandevaporatedto dry-nessusingrotaryevaporator.Thesamplesolutionwaspreparedby accuratelyweighingeachextractanddissolvinginmethanol.Prior totheHPLCinjection,eachsolutionwasfilteredthrougha0.45m nylonmembranefilter.
Stocksolutionsofstandardcompounds1–7werepreparedby accuratelyweighinganddissolvingthecompoundsinacetonitrile toobtainthe final concentrationof 1000g/ml. Working solu-tionsofstandardcompoundswereobtainedbydilutingthestock standardsolutions withacetonitriletoachievethedesired con-centrations.Calibrationcurves were constructedfromthe peak areaversustheamountofthestandardsbyleastsquareregression acrosstherangeof50–1000g/ml.
Resultsanddiscussion
Rhizomesof118Curcumasampleswerecollectedfromvarious
localities of Thailand.Identification wasdone usingthe
ampli-fied fragment length polymorphism (AFLP) markers and their
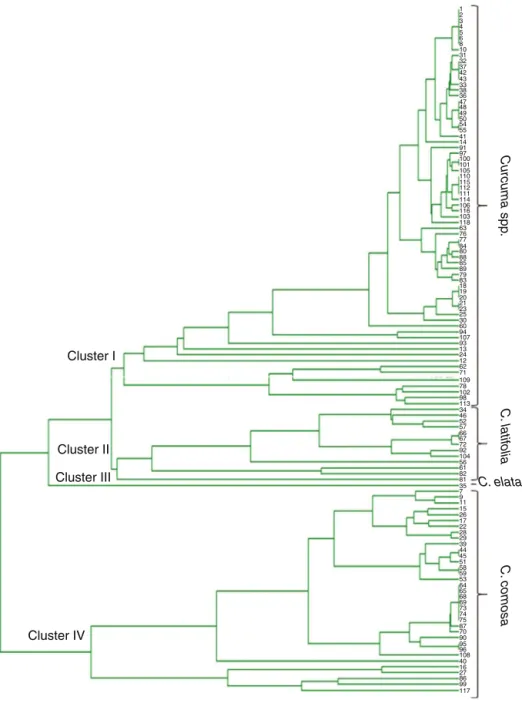
morphologicalcharacteristicsasdescribedinourpreviousstudy (Keeratinijakaletal.,2010).Phylogenetictreeillustratingthe rela-tionshipamong118accessionsasinferredbyAFLPanalysiswas showninFig.1.Thesampleswereclassifiedintofourmajorclusters, i.e.Curcumaspp.(ClusterI),C.latifolia(ClusterII),C.elata(Cluster III),andC.comosa(ClusterIV).Theclusteringoftheaccessionsbased ongeneticsimilaritydidnotcorrelatewiththeregionoforiginof thesamples(Keeratinijakaletal.,2010).
InthecourseofphytochemicalanalysisofCurcumasamples,
45selectedsamplesrepresentingCurcumasp.,C.latifolia,andC. comosawerecultivatedattheNationalCornandSorghumResearch Center,Thailand.Allsamplesweregrownatthesamecondition
and harvested at theage of 8 months.C. elata, a knownplant
containingtoxiccompoundzederone(5)(Pimkaewetal.,2013), hasbeenneglectedduetoitslowpotentialonfurther
develop-ment.Characteristic majorcompounds wereisolated, andtheir
structureselucidatedbyNMRandMSanalyses.Theyhavebeen
identifiedasgermacrone(1),furanodienone(2),curzerenone(3), curdione(4), zederone (5), 1,7-diphehyl-(6E)-6-hepten-3-ol (6), and1,7-diphenyl-(4E,6E)-4,6-heptadien-3-ol(7).Compounds1–5 areclassifiedassesquiterpenoidswhilecompounds6–7are diaryl-heptanoids.
Comparativeanalysisofthemajorcomponentswasdoneusing
high-performanceliquidchromatography(HPLC)technique
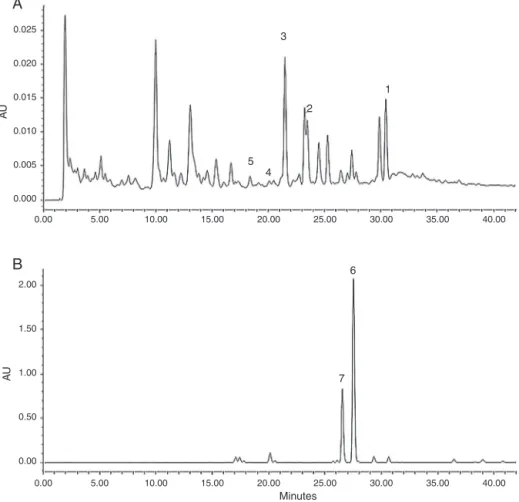
cou-pledwithdiodearraydetector(DAD).UV-spectraobtainedfrom DADcouldconfirmthespecificityofanalytes.Onthebasisofthese findings,twoclear-cutphytochemicalaccumulationtrendscould bedistinguished(Fig.2).Diarylheptanoidphytoestrogens6–7were foundinallsamplesofC.comosa(clusterIV)butnotdetectedinC. latifoliaandCurcumasp.(clusterI–III).Sesquiterpenoids1–5were foundinC.latifoliaandCurcumaspp.(clusterI–III)butnotdetected inC.comosa(clusterIV).Aknowntoxiccompoundzederone(5) wasfoundinallsamplesofC.latifoliaandCurcumaspp.butnot
detectedinC.comosa.Compound
1,7-diphenyl-(6E)-6-hepten-3-one (D2) hasnot been analyzedin this study. However, it has
beenfoundtogetherwithcompounds6–7invarioussamplesof
C.comosa(Suksamrarnetal.,2008).
Sesquiterpenoids1–5werenotfoundinC.comosainourstudy. Since“WanChakModLoog”inThailandcanbeassignedinto vari-ousCurcumaspecies,taxonomicidentificationshouldbecarefully doneduetothevariationinmorphologydependingoncultivation andgeographicaldifference(SoontornchainaksaengandJenjittikul, 2010).However,severalcultivatedplantsinThailandwere mis-takenforsimilarplantspeciessuchasCurcumasp.orC.latifolia insteadofC.comosawhichcontainsthetoxiccomponentzederone (5). The recommendation on using correct plant materials for specificmedicinalpurposesandlarge-scaleproductionshouldbe made.
Despitethecomprehensiveoverviewofvariousphytochemical
V.Keeratinijakal,S.Kongkiatpaiboon/RevistaBrasileiradeFarmacognosia27(2017)290–296 293 Cluster I 1 2 3 4 5 6 8 10 31 32 37 42 43 33 38 36 47 48 49 50 54 55 41 14 91 97 100 101 105 110 115 112 111 114 106 116 103 118 63 76 77 84 80 88 85 89 79 83 18 19 20 21 23 25 30 60 94 107 93 13 24 12 62 71 109 78 102 98 113 34 46 52 57 66 67 72 92 104 56 61 82 81 35 7 9 11 15 26 17 22 28 29 39 44 45 51 58 59 53 64 65 68 69 73 74 75 87 70 90 95 96 108 40 16 27 86 99 117 Cluster II Cluster III Cluster IV C . comosa C . latif olia Curcuma spp . C. elata
Fig.1.Phylogenetictreeillustratingtherelationshipamong118accessionsasinferredbyAFLPanalysis.
1–7in Curcuma sampleswereshown in Table2.However,the methodhasnotbeenvalidatedandfurtheroptimizationto maxi-mizetheseparationandspeedforroutineanalysisisinprogress. Theco-chromatographicallyquantitativeanalyzescouldenablethe comparisonofeachsample.Amongstthesamplestudy,C.comosa variety29gavehighestcontentof1,7-diphenyl-(6E)-6-hepten-3-ol (6)andvariety15gavehighestcontentof 1,7-diphenyl-(4E,6E)-4,6-heptadien-3-ol(7)withoutdetectabletoxicsesquiterpenoids 1–5.
“Wan Chak Mod Loog” in the different cultivars of
Thai-land has been assigned to different C. comosa, C. elata, C.
latifolia,andCurcuma sp.(Soontornchainaksaengand Jenjittikul, 2010; Keeratinijakaletal.,2010).Theyhave beenusedas food supplementandThaitraditionalmedicinefortreatmentof
abnor-mality of the uterus and ovarian hormone deficit. The present
study demonstrates the phytoestrogenic diarylheptanoids and
sesquiterpenoids-containingspecieswhichcouldshedsomelight onthecorrectusesoftheseplants.Ourresultscouldbeusedasa
Table1
Calibationcurvesofcompounds(1)–(7).
Compounds Equation Correlationcoefficient(r2)
V.
Keeratinijakal,
S.
Kongkiatpaiboon
/
Revista
Brasileira
de
Farmacognosia
27
(2017)
290–296
Table2
Percentageofcompounds1–7inCurcumaspp.
Species SampleNo. Content(mg/g)
1 2 3 4 5 6 7
C.comosa 7 – – – – – 10.5321 11.0490
9 – – – – – 13.5346 12.1291
11 – – – – – 11.4672 8.7025
15 – – – – – 13.4491 16.1910
16 – – – – – 13.7456 11.7699
17 – – – – – 12.6640 10.5897
22 – – – – – 10.9100 13.0040
26 – – – – – 9.4777 13.9868
27 – – – – – 10.8922 13.7833
28 – – – – – 11.8533 10.4879
29 – – – – – 17.8707 13.9429
39 – – – – – 11.8671 12.7361
40 – – – – – 13.9232 13.7214
45 – – – – – 10.8725 12.8123
51 – – – – – 10.4936 11.7267
53 – – – – – 7.7451 6.9547
58 – – – – – 8.9132 9.6968
65 – – – – – 12.7290 11.3671
87 – – – – – 10.4617 9.6654
90 – – – – – 9.4295 6.6308
96 – – – – – 10.1766 7.9710
99 – – – – – 12.2783 13.3476
108 – – – – – 11.4772 10.4794
117 – – – – – 12.3013 10.6693
C.latifolia 104 2.8098 0.0096 0.0772 0.0807 11.5061 – –
Curcumasp. 13 1.7880 – – – 13.2415 – –
14 0.8305 – – – 10.6561 – –
21 3.4354 – – – 9.4357 – –
24 0.2527 – – – 2.5481 – –
25 5.7551 – – – 7.9040 – –
30 3.5471 – – – 5.7558 – –
47 2.2337 – – – 11.3616 – –
60 1.4745 – – – 13.2356 – –
63 2.6914 – – – 10.4765 – –
77 5.8431 – 0.0906 0.0799 14.5061 – –
V.Keeratinijakal,S.Kongkiatpaiboon/RevistaBrasileiradeFarmacognosia27(2017)290–296 295
0.025
0.020
0.015
0.010
0.005
0.000
0.00
2.00
1.50
1.00
0.50
0.00
5.00 10.00 15.00
A
B
20.00 25.00
Minutes 7
6 4
5
2
1 3
AU
AU
30.00 35.00 40.00
0.00 5.00 10.00 15.00 20.00 25.00 30.00 35.00 40.00
Fig.2.HPLCchromatographicfingerprintof(A)Curcumalatifoliaextract(sampleNo.104)and(B)C.comosaextract(sampleNo.44).Compoundsidentification:1(retention time,tR30.7min)germacrone;2(tR23.4min)furanodienone;3(tR21.2min)curzerenone;4(tR19.8min)curdione;5(tR18.4min)zederone;6(tR22.7min)
1,7-diphenyl-(6E)-6-hepten-3-ol;7(tR21.8min)1,7-diphenyl-(4E,6E)-6-heptadien-3-ol.HPLCcondition:Agilent1100series,column:SorbaxRP-C18(250×4.6mmi.d.,5m),mobile
phase:(A)waterand(B)acetonitrile,gradientelutionsystem:50–90%(B)in(A)for25minand90%(B)in(A)for5min,flowrate1ml/min,ambienttemperature,injection volume10l,detection260nm.
guidelineforconsumersaswellasfarmerswhomustusetheright cultivars forthe rightmedicinalpurposes.Moreover,C. comosa variety29and15providedthehighestdiarylheptanoidscontents amongtheothersampleswhichcouldbeusedforbreedingand furtherdevelopmentaspharmaceuticalproducts.
Authors’contributions
VKcontributionincludedcollectingsamples,designingand per-forminglaboratorywork,analyzing theresults,and supervision of thelaboratorywork. SKcontributionincluded analyzingthe resultsandpreparingthepaper.Alltheauthorshavereadthefinal manuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
ThisworkwassupportedbytheNationalResearchCouncilof Thailand(NRCT)andNationalCenterforAgriculturalBiotechnology (NCAB).
References
Bhukkhai,K., Suksen,K., Bhummaphan,N.,Janjorn,K.,Thongon,N., Tantikan-layaporn,D.,Piyachaturawat,P.,Suksamrarn,A.,Chairoungdua,A.,2012.A
phytoestrogendiarylheptanoidmediatesestrogenreceptor/Akt/glycogen syn-thasekinase3protein-dependentactivationoftheWnt/-cateninsignaling pathway.J.Biol.Chem.287,36168–36178.
Hariyama,K.,Gao,J.F.,Ohkura,T.,Kawamata,T.,Litaka,Y.,Guo,Y.T.,Inayama,S., 1991.Aseriessesquiterpeneswitha7␣-isopropylsidechainandrelated com-poundsisolatedfromCurcumawenyujin.Chem.Pharm.Bull.39,843–853.
Hikino,H.,Agatsuma,K.,Takemoto,T.,1968.Structureofcurzerenone, epicurz-erenoneandisofranogermacrene(curzerene).TetrahedronLett.9,2855–2858.
Hikino, H.,Konno, C.,Agatsuma, K., Takemoto,T., 1975. Sesquiterpenes. Part XI VII. Structure, configuration,conformation and thermal rearrangement offuranodienone,isofuranodienone,curzerenone,epi-curzerenoneand pyro-curzerenonesesquiterpenesofCurcumazedoaria. J.Chem.Soc.Perk.T.11, 478–484.
Intapad,S.,Suksamrarn,A.,Piyachaturawat,P.,2009.Enhancementofvascular relaxationinrataortabyphytoestrogensfromCurcumacomosaRoxb.Vasc. Pharmacol.51,284–290.
Intapad,S.,Saengsirisuwan,V.,Prasannarong,M.,Chuncharunee,A.,Suvitayawat, W.,Chokchaisiri,R.,Suksamrarn,A.,Piyachaturawat,P.,2012.Long-termeffect ofphytoestrogensfromCurcumacomosaRoxb.onvascularrelaxationin ovariec-tomizedrats.J.Agric.FoodChem.60,758–764.
Jantaratnotai,N.,Utaisincharoen,P.,Piyachaturawat,P.,Chongthammakun,S., San-varinda,Y.,2006.InhibitoryeffectofCurcumacomosaonNOproductionand cytokineexpressioninLPS-activatedmicroglia.LifeSci.78,571–577.
Keeratinijakal,V.,Kladmook,M.,Laosatit,K.,2010.Identificationand characteri-zationofCurcumacomosaRoxb.,phytoestrogens-producingplant,usingAFLP markersandmorphologicalcharacteristics.J.Med.Plant.Res.4,2651–2657.
Niumsakul,S.,Hirunsaree,A.,Wattanapitayakul,S.,Junsuwanitch,N.,Prapanupun, K.,2007.AnantioxidativeandcytotoxicsubstanceextractedfromCurcuma comosaRoxb.J.ThaiTrad.Altern.Med.5,24–29.
Phupatanapong,L.,Chayamarit,K.,Butaweekhun,T.,2001.ThaiplantnamesbyTem Smitinand,2nded.Prachachon,Bangkok,pp.160.
Pimkaew,P.,Suksen,K.,Somkid,K.,Chokchaisiri,R.,Jariyawat,S.,2013.Zederone, asesquiterpenefromCurcumaelataRoxb,ishepatotoxicinmice.Int.J.Toxicol. 32,454–462.
Piyachaturawat,P.,Ercharuporn,S.,Suksamrarn,A.,1995b.Estrogenicactivityof Curcumacomosaextractinrats.AsiaPac.J.Pharmacol.10,121–126.
Piyachaturawat, P., Teeratagolpisa, C., Toskulkao, C., Suksamrarn, A., 1995c.
HypolipidemiceffectofCurcumacomosainmice.Artery22,233–241.
Piyachaturawat,P.,Timinkul,A.,Chauncharunee,A.,Suksamrarn,A.,1998.Growth suppressingeffectofCurcumacomosaextractonmalereproductiveorgansin immaturerats.Pharm.Biol.36,44–49.
Piyachaturawat,P.,Charoenpiboonsin,J.,Toskulkao,C.,Suksamrarn,A.,1999a.
ReductionofplasmacholesterolbyCurcumacomosaextractin hypercholes-terolaemichamsters.J.Ethnopharmacol.66,199–204.
Piyachaturawat,P.,Timinkul,A.,Chaunchararunee,A.,Suksamrarn,A.,1999b.Effect ofCurcumacomosaextractonmalefertilityinrats.Pharm.Biol.37,22–27.
Qu,Y.,Xu,F.,Nakamura,S.,Matsuda,H.,Pongpiriyadacha,Y.,Wu,L.,Yoshikawa,M., 2009.SesquiterpenesfromCurcumacomosa.J.Nat.Med.63,102–104.
Shibuya,H.,Hamamoto,Y.,Cai,Y.,Kitakawa,I.,1987.Areinvestigationofthe struc-tureofzederone,afuranagermacrane-typesesquiterpernefromzedoary.Chem. Pharm.Bull.35,924–927.
Soontornchainaksaeng,P.,Jenjittikul,T.,2010.Chromosomenumbervariationof phytoestrogen-producingCurcuma(Zingiberaceae)fromThailand.J.Nat.Med. 64,370–377.
Sodsai,A.,Piyachaturawat,P.,Sophasan,A.,Suksamrarn,A.,Vongsakul,M.,2007.
SuppressionbyCurcumacomosaRoxb.ofpro-inflammatorycytokinesecretion inphorbol-12-myristate-13-acetatestimulatedhumanmononuclearcells.Int. Immunopharmacol.7,524–531.
Su,J.,Sripanidkulchai,K.,Wyss,J.M.,Sripanidkulchai,B.,2010.Curcumacomosa improveslearningandmemoryfunctiononovariectomizedratsinalong-term Morriswatermazetest.J.Ethnopharmacol.130,70–75.
Su,J.,Sripanidkulchai,B.,Sripanidkulchai,K.,Piyachaturawat,P.,Wara-aswapati, N.,2011.EffectofCurcumacomosaandestradiolonthespatialmemoryand hippocampalestrogenreceptorinthepost-trainingovariectomizedrats.J.Nat. Med.65,57–62.
Su,J.,Sripanidkulchai,K.,Hu,Y.,Sripanidkulchai,B.,2012a.Curcumacomosa pre-ventstheneuronlossandaffecttheantioxidativeenzymesinhippocampusof ethanol-treatedrats.Pak.J.Biol.Sci.15,367–373.
Su,J.,Sripanidkulchai,K.,Suksamrarn,A.,Hu,Y.,Piyachaturawat,P.,Sripanidkulchai, B.,2012b.Pharmacokineticsandorgandistributionofdiarylheptanoid phytoes-trogensfromCurcumacomosainrats.J.Nat.Med.66,468–475.
Suksamrarn,A.,Eiamong,S.,Piyachaturawat,P.,Byrne,L.T.,1997.A phloracetophe-noneglucosidewithchloreticactivityfromCurcumacomosa.Phytochemistry 45,103–105.
Suksamrarn,A.,Ponglikitmongkol,M.,Wongkrajang,K.,Chindaduang,A., Kitti-danairak,S.,Jankam,A.,Yingyongnarongkul,B.,Kittipanumat,N.,Chokchaisiri, R., Khetkam, P., Piyachaturawat, P., 2008. Diarylheptanoids, new phy-toestrogens from the rhizomes of Curcuma comosa: isolation, chemical modificationand estrogenic activityevaluation.Bioorgan. Med.Chem.16, 6891–6902.
Tantikanlayaporn, D.,Robinson, L.J., Suksamrarn, A., Piyachaturawat, P., Blair, H.C., 2013a.A diarylheptanoid phytoestrogenfrom Curcumacomosa, 1,7-diphenyl-4,6-heptadien-3-ol,accelerateshumanosteoblastproliferationand differentiation.Phytomedicine20,676–682.
Tantikanlayaporn,D.,Wichit,P.,Weerachayaphorn,J.,Chairoungdua,A., Chun-charunee,A.,Suksamrarn,A.,Piyachaturawat,P.,2013b.Bonesparingeffectof anovelphytoestrogendiarylheptanoidfromCurcumacomosaRoxb.in ovariec-tomizedrats.PLoSOne8(11),e78739.
Weerachayaphorn, J.,Chuncharunee, A., Mahagita, C., Lewchalermwongse, B., Suksamrarn, A., Piyachaturawat, P., 2011. Aprotective effect of Curcuma comosaRoxb.onbonelossinestrogendeficientmice.J.Ethnopharmacol.137, 956–962.
Winuthayanon,W.,Piyachaturawat,P.,Suksamrarn,A.,Ponglikitmongkol,M.,Arao, Y.,Hewitt,S.C.,Korach,K.S.,2009a.Diarylheptanoidphytoestrogensisolated fromthemedicinalplantCurcumacomosa:biologicalactionsinvitroandinvivo indicateestrogenreceptor-dependentmechanisms.Environ.HealthPersp.117, 1155–1161.
Winuthayanon,W.,Suksen,K.,Boonchird,C.,Chuncharunee,A.,Ponglikitmongkol, M.,Suksamrarn,A.,Piyachaturawat,P.,2009b.Estrogenicactivityof diarylhep-tanoidsfromCurcumacomosaRoxb.requiresmetabolicactivation.J.Agric.Food Chem.59,840–845.
Xu,F.,Nakamura,S.,Qu,Y.,Matsuda,H.,Pongpiriyadacha,Y.,Wu,L.,Yoshikawa,M., 2008.StructuresofnewsesquiterpenesformCurcumacomosa.Chem.Pharm. Bull.56,1710–1716.