w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Anatomy
and
histochemistry
of
leaves
and
stems
of
Sapium
glandulosum
Evelyn
Assis
de
Andrade
a,
Daniela
Gaspardo
Folquitto
b,
Lívia
Eidam
Camargo
Luz
c,
Kátia
Sabrina
Paludo
d,
Paulo
Vitor
Farago
b,
Jane
Manfron
Budel
b,∗ aCursodeFarmácia,UniversidadeEstadualdePontaGrossa,PontaGrossa,PR,BrazilbDepartamentodeCiênciasFarmacêuticas,UniversidadeEstadualdePontaGrossa,PontaGrossa,PR,Brazil cDepartamentodeFarmácia,UniversidadeEstadualdeMaringá,Maringá,PR,Brazil
dDepartamentodeBiologiaEstrutural,MoleculareGenética,UniversidadeEstadualdePontaGrossa,PontaGrossa,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received10August2016 Accepted10January2017 Availableonline16February2017
Keywords:
Druses Euphorbiaceae Pau-leiteiro
Pharmacobotanicalstudy Latex
Qualitycontrol
a
b
s
t
r
a
c
t
SapiumbelongstoEuphorbiaceaefamilyandcomprises23species.Sapiumglandulosum(L.)Morongis popularlyknowninBrazilas“pau-leiteiro”and“leitosinha”anditisusedintraditionalmedicineto cicatrisation.Itsleafextractshaveshownanalgesic,anti-inflammatoryandantibacterialactivities.The preliminarysetofpharmacognostictoolsusedforqualityassessmentofmedicinalplantpartsis macro-andmicro-anatomyandS.glandulosumhasnotanatomicalandhistochemicaldescription.Thustheaim ofthisstudywastoinvestigatetheanatomicalandhistochemicalcharacteristicsoftheleafandstem ofS.glandulosumasameansofprovidinginformationforqualityassessmentofherbalindustry.The leavesandstemswereinvestigatedbyemployingfieldemissionscanningelectronmicroscopy,light microscopy,andhistochemistrytechniques.TheanalysisshowedthatS.glandulosumhadthefollowing anatomicalfeatures:dorsiventralandamphistomaticleaves;paracyticstomata;tabularcrystaldruses; non-articulatedandbranchedlaticifers;midrib’sbiconvexshapewithvascularsystemsinopenarcwith invaginatedends;petiolewitharoundshapeandslightconcavityontheadaxialside;sixcollateral vascularbundlesinU-shapedorganisation;acircularstemshapeandasclerenchymatousring.Inthe histochemicaltestslipophiliccomponentswerefoundincuticleandinthelatex;phenoliccompounds weremetinthemesophyllandinthelatex;starchgrainswerefoundintheparenchymatoussheath; lignifiedelementsweremetinthesclerenchymatousringinthecortexandintheperivascular scle-renchymatouscaps,beyondinthevesselelements.Thesefeaturesarehelpfulwhenconductingaquality controlprocess.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
SapiumJacq. isone ofthemostimportant genusof Euphor-biaceae.It consistsof23acceptedspecies(ThePlantList,2015) anddeserves consideration becauseof thecomplexityinvolved indelimitingitsspecies(Seccoetal.,2012).It isformedmainly byneotropicalspeciesandisdistributedinfields,savannas, sea-sonalforests,rainforestsandwoodlands(SátiroandRoque,2008;
PscheidtandCordeiro,2012).
This genus presents several species that are used in popu-larmedicine,suchasS.chihsinianumS.K.Lee,S.discolor(Champ. ex Benth) Muell. Arg.,S. rotundifolium Hemsl., and S. sebiferum
(L.)Roxb,whichareusedmainlytocicatrisation(AlMuqarrabun
∗ Correspondingauthor.
E-mail:jane@uepg.br(J.M.Budel).
etal.,2014).Somespeciesof Sapiumhavebeenchemicallyand pharmacologicallystudied.Extractsandsinglecomponentsfrom thisgenuswerereportedtohavepromisingbiologicalactivities suchasantioxidant,antimicrobial,andcytotoxiceffects(Hajduand
Hohmann,2012;AlMuqarrabunetal.,2014).
Sapiumglandulosum(L.)Morong,whichispopularlyknownin Brazilas“pau-leiteiro”and“leiteiro”,isatreethatcanreach3–8m inheightandisamongthemostpolymorphicspeciesofSapium.Itis usedintraditionalmedicinetotreathernias(HajduandHohmann,
2012;AlMuqarrabunetal.,2014)anditsusehasbeenpotentially
recommendedfortherecoveryofdegradedareas(Ferreiraetal.,
2009).
Theleavesof S. glandulosumcontainanthracene derivatives, monoterpenes,tanninsandflavonoids(daSilvaetal.,2011,2012). Thisspeciesislatex-bearingandthelatexhasproteinswith con-siderableproteolyticactivity.Thisactivityisnotablyinhibitedby aserineproteaseinhibitor(Sobottkaetal.,2014).Theleafextracts
http://dx.doi.org/10.1016/j.bjp.2017.01.001
Classifying medicinalplants isa serious problembecauseof theircommonnames.Asinglemedicinalspeciesfrequentlyhas anumberofpopularnamesandapopularnamecanoccasionally beusedforarangeofplants(Uptonetal.,2011).Somespeciesof
Sapium,suchasS.glandulosum,S.arbutum(Müll.Arg.)Huberand
S.sellowianum(Müll.Arg.)KlotzschexBaill(Agraetal.,2008),are popularlyknowninBrazilas“pau-leiteiro”,“leiteiro”or “burra-leiteira”.Inthiscontextthemostimportantconsequenceinregard totheuseofinappropriatefolknamesisthesubstitutionof ther-apeuticand safeherbsby toxicvegetable species(Uptonetal.,
2011).
Thepreliminarysetofpharmacognostictoolsusedforquality assessmentofmedicinalplantpartsismacro-andmicro-anatomy
(Uptonetal.,2011).Consequently,theaimofthisstudywasto
investigatetheanatomicalandhistochemicalcharacteristicsofthe leafandstemofS.glandulosumasameansofprovidinginformation forqualitycontrolintheherbalindustry.Furthermore,thereare nopreviouspapersintheliteratureaboutthepharmacobotanical characteristicsofthistaxon.
Materialsandmethods
Plantmaterial
The leaves and stems of Sapium glandulosum (L.) Morong, Euphorbiaceae,werecollectedfromgrownspecimensinopenand sunnyareasintheCamposGeraisregionofParaná(24◦18′Sand
49◦37′W),BrazilinOctober2013.Matureleavesandstems(atleast
tensamples)obtainedfromthesixthnodeandbelow(median, intercostalandmarginregions),aswellasstemfragmentsfrom 5to15cmfromtheshootwerepreparedforthe pharmacobotan-icalassays.Theplantmaterialcontaininginflorescenceswasused toprepareavoucherspecimen,whichwasidentifiedbyOsmardos SantosRibasandstoredattheMuseuBotânicodeCuritibaunder thenumber390589MBM.
Pharmacobotanicalassays
TheleavesandstemsofS.glandulosumwereplacedinasolution
ofFAA70(Johansen,1940),andstoredin70%ethanol(Berlynand
Miksche,1976).Fortheexaminationofleafandstemmaterial
free-handlongitudinalandcross-sectionswereprepared.Intheleaves itwasincludedthemidrib,interneuralregions,andlateralveins. ThesematerialswerestainedusingAstrablueandbasicfuchsine
(Roeser,1972)andtoluidineblue(O’Brienetal.,1964)toobtain
semi-permanentslides.Thediaphanisationoftheleaveswas per-formedbyfollowingthetechniqueofFuchs(1963).Forthecrystals descriptionsHeetal.(2012)wereused.
Histochemicaltests
Thefollowingstandardsolutionswereemployedinthe histo-chemicaltests:methylenebluetotestformucilage(Oliveiraetal., 2005);hydrochloricphloroglucintorevealtracesoflignin(Sass, 1951);SudanIIIfortestinglipophiliccompounds(Foster,1949); Hoepfner–Vorsatztest, modifiedby Reeve(1951)(aqueous 10% sodiumnitrate,aqueous10%aceticacid,aqueous10%ureaand,2N NaOH)andferricchloridetotestforphenolicsubstances(Johansen, 1940);Bouchardatreactivefornitrogencompounds(Borio,1959); methylenebluetotestmucilage(Oliveiraetal.,2005)and iodine-iodidetorevealstarch(BerlynandMiksche,1976).
PhotomicrographswerecapturedusingaOlympusCX31light microscopethatwasequippedwithaC7070digitalcamera.The semi-permanentandhistochemicaltestslideswerethenanalysed
Fieldemissionscanningelectronmicroscopy(FESEM)and energy-dispersiveX-rayspectroscopy(EDS)
Forthefieldemissionscanningelectronmicroscopy(Mira3 Tes-can)freshleavesandstemswereused.Thesamplesweresubmitted inhighvacuumwithhighacceleratingvoltage(15kV).Thismethod requiredthesamplestobepreviouslydehydratedusingincreasing amountsofethanolthendriedinacriticalpointdryer.Afterwards, theyweresubmittedtometallisationwithgold(Quorum,modelo SC7620).QualitativeX-raymicroanalyseswereperformedon cer-taincrystalsandincellswithoutcrystals(control)usinganEDS machine (Mira 3Tescan)onthe samevariable-pressure micro-scope.Thisprocedurewascarriedoutatthemulti-userlaboratory (LABMU)ofUEPG.
Resultsanddiscussion
TheleavesofS.glandulosum(Fig.1A,B),infrontalview,showed epidermalcellswithstraighttoslightwavyanticlinalwalls(Fig.1C, F), which were relatively thin on both sides. The leaves were amphistomaticandtheparacyticstomatawereobserved predom-inantlyontheabaxialside(Fig.1C–E).Ontheadaxialside,they appeared only near themidrib as observed in Fig. 1F, G. They measured35minlengthonaverageandthestriatecuticlewas
tangentiallypositionedinthesubsidiarycells(Fig.1C–E).Metcalfe
andChalk(1950)reportedparacyticstomataintheEuphorbieae
tribe.ValleandKaplan(2000)reportedthatS.glandulosumhad amphistomaticleaves,whileS.sellowianum(Müll.Arg.)Klotzschex Baill.hadhypostomaticleaves.Theseauthorsaffirmedthatthe dis-tributionofstomatawasataxonomicfeaturethathelpstoseparate thesetwospecies.
Incross-section,theepidermiswasuniseriateandthecellswere largerontheadaxialside.Thecuticlewassmoothandthinand reactedwithSudanIIIinthehistochemicaltest(Fig.1H).Druses werefoundintheepidermalcells(Fig.1H).Themesophyllwas dor-siventralandwasformedbyonelayerofpalisadeparenchymaand abouteightlayersofspongyparenchyma.Smallcollateralvascular bundles were immersed in the mesophyll and they were sur-roundedbyaparenchymatoussheath.Druseswerealsoobserved inthemesophyll(Fig.1H).
Phenoliccompoundsaresecondarymetabolitesresponsiblefor adaptationandresistancetohostileenvironmentfactors.Theyare implicatednotonlyinthedefensemechanismsofplantsagainst fungalpathogensbutalsoagainstinsectherbivores(Lattanzioetal., 2006).Inthepresentstudy,phenoliccompoundsreactedpositively withferricchlorideandHoepfner–Vorsatztestandtheyarefound inthemesophyll.
Themidrib,intransection,wasbiconvex;however,the convex-ity wasmore conspicuousontheabaxial surface(Fig.2A).The epidermisis uniseriate and it is covered bya striate and thick cuticle.ThecuticlereactedwithSudanIII(Fig.2C).Thecuticleis themostimportantbarrieragainstuncontrolledwaterlossfrom leaves,stems,fruitsandotherpartsofhigherplants(Riedererand
Schreiber,2001).Cutinisthemaincomponentofthecuticleandis
alipophilicpolymerthatisdepositedinandthetopoftheouter wallepidermalcells(Uptonetal.,2011).Cuticleornamentationis oneofthemostusefultaxonomiccharacteristicsofepidermisin leavesappearingasstriations,ridges,orpapillae(Barthlottetal.,
1998;Uptonetal.,2011).
A
B
C
D
E
G
H
F
ab
ad
st
ct
st
wa
ct
st
st
ct
st
md
st
pp
ep
ct
dr
sp
Fig.1. Sapiumglandulosum(L.)Morong,Euphorbiaceae.(A)Aspectofaerialvegetativeorgans,inhabit.(B)Leaves,showingabaxial(ab)andadaxial(ad)sides.(C)Abaxial sideofepidermisinfrontalview,showingstomata(st)andstriatecuticle(ct).(D)Abaxialsideofepidermisinfrontalview,showingstomata(st)andstriatecuticle(ct) (FESEM–fieldemissionscanningelectronmicroscope).(E)Detailofthestomata(st),epicuticularwaxes(wa)andstriatecuticle(st),showingmeasureofthestomatainthe abaxialepidermis–FESEM.(F)Adaxialsideofepidermisinsurfaceview,indicatingstomata(st).(G)Adaxialsideofepidermisinsurfaceview,indicatingstomata(st)near themidrib(md)–FESEM.(H)Leafincross-sectionindicatingcuticle(ct),druses(dr),epidermis(ep),palisadeparenchyma(pp),spongyparenchyma(sp).Scalebar=1cm (A,B),10m(E),50m(C,D,F,H),and100m(G).
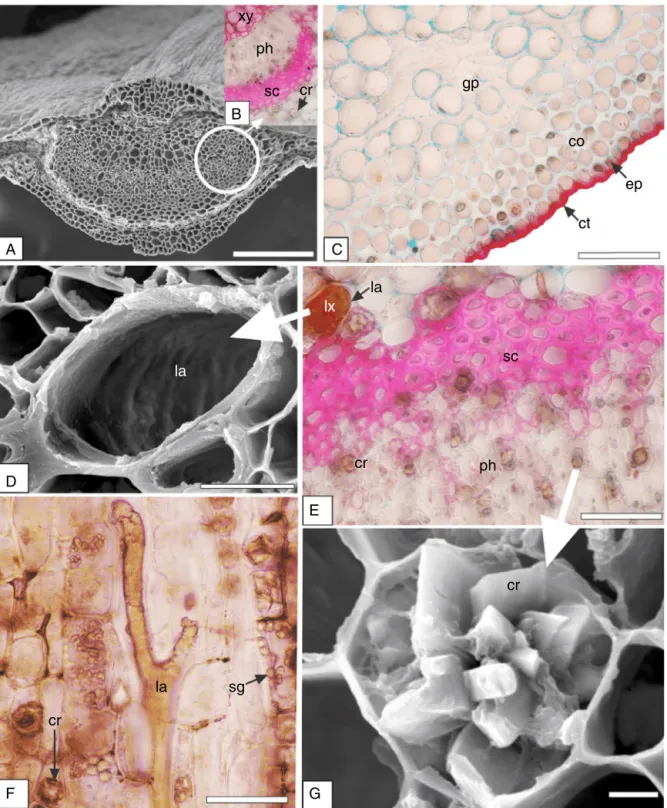
openarcwithinvaginatedends,whichwassurroundedbya scle-renchymaticsheath(Fig.2A,B).Theorganisationofvascularsystem isrelevantfeatureofspeciescharacterisationanddifferentiation
(Almeidaetal.,2017;Bobeketal.,2016).Inthemidriboftheleaf
ofEuphorbiaceae,theorganisationofthevasculartissueswas
vari-able(Gaucher,1902).ThevascularsystemorganisationcanhelpS.
glandulosumidentification.
Severaldruseswereevidentinthegroundparenchyma,mainly nearthesclerenchymaticsheath(Fig.2B,E,G).Drusesare consid-eredclustercrystalsandareformedbyaggregateshavingseveral sidesand sharppoints(Uptonet al.,2011)asblockys,tabulars, styloids,andtetrahedralcrystals(Heetal.,2012).Inthepresent
ph
sc
cr
A
D
F
G
E
B
C
gp
co
ct
ep
la
la
sc
lx
cr
ph
cr
sg
la
cr
Fig.2.Sapiumglandulosum(L.)Morong,Euphorbiaceae–Midrib.(A)Generalaspectincross-section(FESEM).(B)Detailofthevascularsysteminreactionwithhydrochloric phloroglucin,indicatingcrystal(cr),sclerenchyma(sc),phloem(ph)andxylem(xy).(C)Detailoftheabaxialside,showingcollenchyma(co),cuticle(cu)inreactionwith SudanIII,epidermis(ep),groundparenchyma(gp).(D)LaticiferinFESEM.(E)Phloem(ph)region,evidencingcrystals(cr),sclerenchyma(sc),laticifers(la)withlatex(lx). (F)LaticiferinlongitudinalsectioninreactionwithHoepfner–Vorsatzmodifiedreagent,crystals(cr)andstarchgrains(sg).(G)Tabularcrystaldruse(cr)-FESEM.Bar=2m
(G),10m(D),50m(C,E,F),100m(B),and200m(A).
Pompert(1989)reportedthatthelaticifersweresmallerandless
frequentinS.haematospermumthaninS.longifolium.
Accordingto Demarcoet al. (2013), Euphorbiaceae presents non-articulatedbranchingandarticulatedanastomosinglaticifers. These authorsreportedthat S. haematospermumpresented two articulatedlaticiferssystemsin theleafandstem,onethatwas formedof narrowlaticifersand theother shapedby wide lati-cifers.Non-articulatedlaticifersareinitialisedfromsinglecellsat anearlyperiodinseedlinggrowth;articulatedlaticifersareformed
bychainsofcells whose adjoiningwallscanoccasionallybreak down,formingvessels(Rudall,1987).
ep
co
A
C
B
D
sg
cr
xy
ph
E
F
la
la
vb
Fig.3.Sapiumglandulosum(L.)Morong,Euphorbiaceae–Petiole.(A)Generalaspect.(B)Collenchyma(co)andepidermis(ep).(C)Vascularsystem,showingvascularbundle (vb),phloem(ph),xylem(xy)andcrystals(cr).(D)Detailofthestarchgrainsinreactionwithiodine-iodide.(E)Longitudinalsection,showinglaticifer(la)inreactionwith ferricchloride.(F)Longitudinalsectionindicatinglaticifer(la)inreactionwithSudanIII.Bar=25m(D),50m(B,C,E,F),and200m(A).
insectherbivores(Konno,2011).Itisfoundinthevacuoleof secre-torycellsknownaslaticifers,whichincludeacomplexcombination ofcompoundssuchasphenolics,enzymes,terpenes,alkaloids, vita-mins,mucilage,andlipids(Hageletal.,2008;Folquittoetal.,2014; Luzet al.,2015).Latexhasbeenattributedwithcytotoxic(Luz etal.,2015),anti-tumour(Biscaroetal.,2013),anti-ulcer(Costa etal.,2012),andproteinase(Sobottkaetal.,2014)activities,among others.
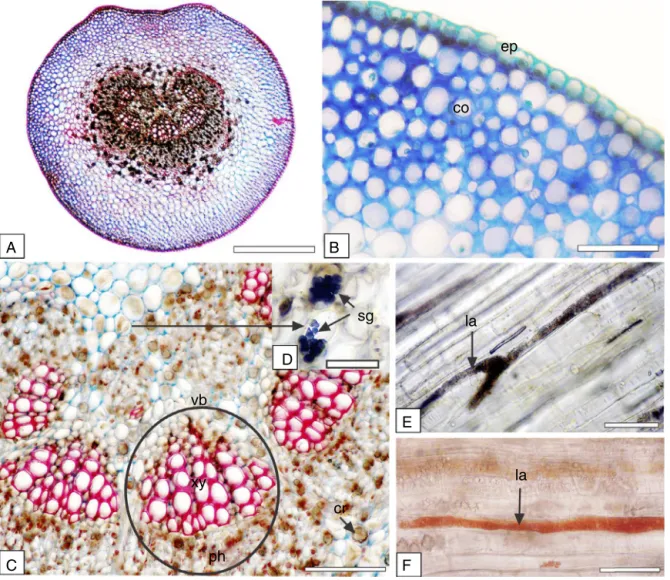
The petiole in the middle part and in cross-section had an almostroundshape,however,withaslightconcavityonthe adax-ialside (Fig.3A).Theepidermishad thesamecharacteristicsas theleafbladeandreactedwithSudanIIIinthemicrochemicaltest. Beneaththeepidermis,therewereabouteightlayersofangular col-lenchyma(Fig.3B).Laticifers(Fig.3E,F)aspreviouslydescribedfor themidrib,couldbeobservednearthevascularbundles.Druses werefoundinthegroundparenchyma.Thevascularsystemwas formedbyaboutsixcollateralvascularbundles(Fig.3C)ina U-shapedorganisation.Almeidaetal.(2016)andBobeketal.(2016)
indicatedtheimportanceoftheshapeandvascularpatternofthe petioleincross-sectionandaffirmedthatthesecharacteristicscan beusedasgoodmarkersinplants.
Starch is widelydistributed throughout plant tissues, but is commonlyfoundinhighestconcentrationsinroots,rhizomes,and fruits(Uptonetal.,2011).Inthepresentstudystarchgrainswere metinthegroundparenchymaofthemidrib(Fig.2F)andinthe
vascularbundlesheathofpetioleandreactedwithiodine-iodide in the histochemical test (Fig. 3D). Not only the presence but alsothestructureofstarchgranulescanbeimportantfortaxon identification(Uptonet al., 2011).In S. glandulosumtheywere very smalland rounded and/orovate and appeared compound aggregatesoftwoormoregranules.
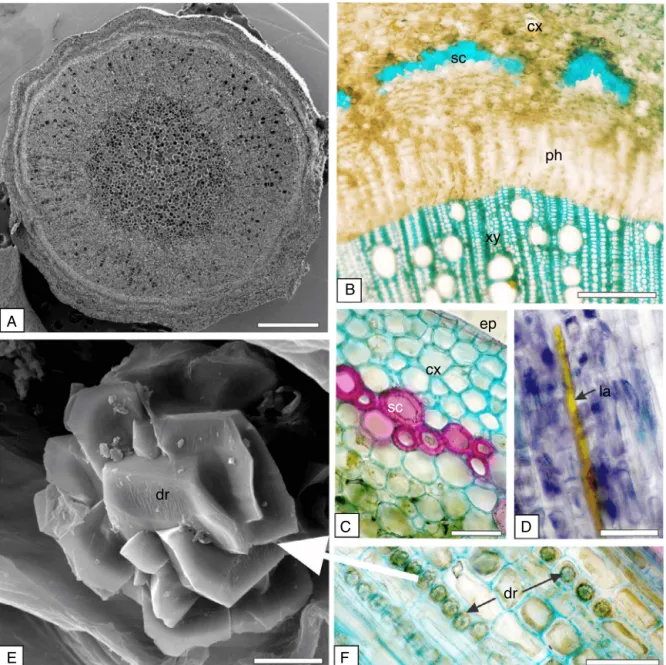
Intransection,thestempresenteda circularshape (Fig.4A). The epidermisappeared in a singleseries withthickened cuti-cle.Therewereseverallayersof cellsin thecortex(Fig.4B,C). Thesclerenchymawasformedbythickenedcellscontaininglignin, leadingtoasclerenchymatousringinthecortex(Fig.4C)andinthe perivascularsclerenchymatouscaps(Fig.4B).Theyreactedwith hydrochloricphloroglucinreagent(Fig.4C)andwithmethylene blue(Fig.4B).Ligninispolymerhighphenolicswhichconferswater resistance,strength,andelasticity.Itisdepositedamongthe cel-lulosemicrofibrilsofprimaryand/orsecondarycellwalls(Upton etal.,2011).Thepresenceofperivascularsclerenchymatouscaps helpedtheS.glandulosumidentification.
Theendodermiswasformedbyalayerofcells.Thevascular cylinderpresented cambia,formingphloemoutwardand xylem inward. As expected,lignin was also found in vessel elements
(Figs.2B,3C,4B).Laticifers,aspreviouslyreportedforthemidrib
sc
ph
xy
ep
la
dr
dr
cx
sc
A
E
F
B
C
D
Fig.4.Sapiumglandulosum(L.)Morong,Euphorbiaceae–Stem.(A)GeneralaspectinFESEM.(B)Cross-section,showingcortex(cx),phloem(ph),sclerenchyma(sc)and xylem(xy).(C)Detailofthecellsofthesclerenchymatousring,cortex(cx)andepidermis(ep).(D)Longitudinalsection,showinglaticifers(la).(E)Tabularcrystaldruses(cr) inFESEM.(F)Cortexshowingseveralcrystals(cr).ScaleBar=2m(E),50m(B–D,F),and200m(A).
Crystalidioblasts were gathered in the stem (Fig.4E,F), as observedinthemesophyll,midribandpetiole.Inthepresentstudy, thecrystalswereanalysed fortheirelementalcomposition and thespectrashowedprominentpeaksforcalcium(27.74%),carbon (16.7%)andoxygen(55.56%),ascanbeseeninFig.5,indicatingthat thesecrystalswereformedofcalciumoxalate.Crystalshavebeen
identifiedinsomestudiesascalciumoxalatebyusingEDS(Heetal.,
2012;Almeidaetal.,2016;Swiechetal.,2016).
The functionsof crystalsin plants are toact as an internal reservoirforcalcium,toprovidetissuerigidity,ionicbalance,to remove calcium, magnesium, oxalicacid, aluminium and other heavymetals,andalsotoactasaprotectivedeviceagainst
for-20
10
0
Ca
O
C
Ca
Spectrum 2
0 2 4 6 8 10 12 14 16 18 keV
cps/eV
aginganimals(FranceschiandNakata,2005;Heetal.,2012;Silva etal.,2014).Thepresenceorabsenceofcrystals,theirtype and theirchemicalcomposition,canbecharacterisedastaxonomic fea-tures(Meric,2009).Withreferencetothechemicalcomposition, excesscalciumishabituallyprecipitatedincalciumsaltssuchas carbonate,citrate,malate,oxalate,phosphate,silicateandsulphate
(WeinerandDove,2003).
Crystals of calcium oxalateare most commonly reported in higherfamiliesandtheyoccurinmostorgansandtissuesinthe vegetablespecies.However,theirsizeandnumberareresponsive tochanges intheconcentration ofcalcium intheenvironment
(Nakata,2003;FranceschiandNakata,2005).Crystalsofcalcium
oxalateareformedfromendogenouslysynthesisedoxalicacidand Catakenfromtheenvironment,andtheyareformedand accumu-latedinspecies-specificmorphologies(Meric,2009).
Anatomicalcharacterisationisaninherentpartofpracticallyall pharmacopoeiasandisoneoftheprimaryidentificationtests nec-essaryfor pharmacopoeialcompliance.Theindividualstructural elementsarecomparativelyfrequentwithinthesameplantorgans, butthewaysinwhichthetissues,elementsandcellsaresetwithin aplantorganpermitsdiagnosistobeperformedandlendsupport tothequalityassessmentofherbaldrugs(Uptonetal.,2011).
Themainanatomicalcharacterswerehighlightedinthis phar-macobotanical study, which was performed to provide more informationaboutthestandardisationoftheS.glandulosumspecies in orderto supportthequality control of this vegetable mate-rial.Thefollowingcharacteristicsarehelpfulwhenconductingthe qualitycontrolprocess:dorsiventraland amphistomaticleaves; paracyticstomata;calciumoxalate tabularcrystal druses; non-articulatedandbranchedlaticifers;biconvexwithvascularsystems inopenarcwithinvaginatedends;petiolewithroundshapeand slightconcavityontheadaxialside;sixcollateralvascularbundles inU-shapedorganisation;circularstemshape,sclerenchymatous ringinthecortexandperivascularsclerenchymatouscaps.
Thehistochemicaltestshowedthepresenceofthelipophilic andphenoliccompoundsinthelatex;phenoliccompoundsinthe mesophyll;starchgrainssmallandroundedandcompound aggre-gatesoftwoormoregranules;lignifiedelementsweremetinthe sclerenchymatousringinthecortexandintheperivascular scle-renchymatouscaps,beyondinthevesselelements.
Authors’contributions
EAA,DGF,LECL andKSPassisted in carryingout the labora-torywork.EAAcontributed incollectingtheplantmaterial and itsidentification.PVFperformedthescanningelectronmicroscopy (FESEM)analysis.JMBcreatedtheproject,supervisedthe labora-torywork,andwrotethepaper.Alltheauthorshavereadthefinal manuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorswouldliketothanktheElectronMicroscopyCentre oftheLABMUattheStateUniversityofPontaGrossaforproviding theFESEMimagesandEDSspectra.
References
Agra,M.F.,Silva,K.N.,Basílio,I.J.L.D.,deFreitas,P.F.,Barbosa-Filho,J.M.,2008.Survey ofmedicinalplantsusedintheregionNortheastofBrazil.Rev.Bras.Farmacogn. 18,472–508.
AlMuqarrabun,L.M.R.,Ahmat,N.,Aris,S.R.S.,2014.Areviewofthemedicinaluses, phytochemistryandpharmacologyofthegenusSapium.J.Ethnopharmacol.155, 9–20.
Almeida,V.P.,Hirt,A.A.,Raeski,P.A.,Mika,B.E.,Justus,B.,dosSantos,V.L.P.,Franco, C.R.C.,dePaula,J.P.,Farago,P.V.,Budel,J.M.,2017.Comparative morphoanatom-icalanalysisofMikaniaspecies.Rev.Bras.Farmacogn.27,9–19.
Barthlott,W.,Neinhuis,C.,Cutler,D.,Ditsch,F.,Meusel,I.,Theisen,I.,Wilhelmi,H., 1998.Classificationandterminologyofplantepicuticularwaxes.Bot.J.Linn. Soc.126,227–236.
Berlyn,G.P.,Miksche,J.P.,1976.BotanicalMicrotechniqueandCytochemistry.Iowa StateUniversity,Ames.
Biscaro,F.,Parisotto,E.B.,Zanette,V.C.,Günther,T.M.F.,Ferreira,E.A.,Gris,E.F., Cor-reia,J.F.G.,Pich,C.T.,Mattivi,F.,WilhelmFilho,D.,Pedrosa,R.C.,2013.Anticancer activityofflavonolandflavan-3-olrichextractsfromCrotonceltidifoliuslatex. Pharm.Biol.51,737–743.
Bobek,V.B.,Heiden,G.,Oliveira,C.F.,Almeida, V.P.,dePaula,J.P.,Farago,P.V., Nakashima,T.,Budel,J.M.,2016.Comparativeanalyticalmicrographsof vas-souras(Baccharis,Asteraceae).Rev.Bras.Farmacogn.26,665–672.
Borio,E.B.L.,1959.LobelialangeanaDusén.Contribuic¸ãoparaoestudo farmacognós-tico.daFaculdadedeFarmáciadaUniversidadedoParaná,Brasil,86pp.Tesepara concursoàdocêncialivredacadeiradefarmacognosia,FaculdadedeFarmácia daUniversidadedoParaná.
Costa,L.L.G.,David,V.C.,Pinto,R.M.C.,Minozzo,B.R.,KozlowskiJr.,V.A.,Campos,L.A., Silva,R.Z.,Beltrame,F.L.,2012.Anti-ulceractivityofSynadeniumgrantiilatex. Rev.Bras.Farmacogn.22,1070–1078.
daSilva,C.H.T.P.,Sobrinho,T.J.S.P.,Saraiva,A.M.,Pisciottano,M.N.C.,deAmorim, E.L.C.,2012.Phytochemicalprofileandantibacterialactivityofbarkandleaves
ofCaesalpiniapyramidalisTul.andSapiumglandulosum(L.)Morong.J.Med.Plants
Res.6,4766–4771.
daSilva,C.H.T.P.,Sobrinho,T.J.S.P.,eCastro,V.T.N.A.,Lima,D.C.A.,deAmorim,E.L.C., 2011.AntioxidantcapacityandphenoliccontentofCaesalpiniapyramidalisTul.
AndSapiumglandulosum(L.)MorongfromNortheasternBrazil.Molecules16,
4728–4739.
Demarco,D.,Castro,M.M.,Ascensão,L.,2013.TwolaticifersystemsinSapium
haematospermum–newrecordsforEuphorbiaceae.Botany91,545–554.
Demarco,D.,Kinoshita,L.S.,Castro,M.M.,2006.Laticíferosarticulados anastomosa-dos:novosregistrosparaApocynaceae.Rev.Bras.Bot.29,133–144.
Ferreira, B.G.A., Zuffellato-Ribas, K.C., Carpanezzi, A.A., Tavares, F.R., Koehler, H.S.,2009.Metodologias deaplicac¸ãodeAIBnoenraizamento deestacas semilenhosasdeSapiumglandulatum(Vell.)Pax.Rev.Bras.PlantasMed.11, 196–201.
Folquitto,D.G.,Budel,J.M.,Pereira,C.B.,Brojan,L.E.F.,Folquitto,G.G.,Miguel,M.D., Silva,R.Z.,Miguel,O.G.,2014.Analyticalmicrographyandpreliminary phyto-chemistryoftheleavesandstemsofLobeliaexaltataPohl.(Campanulaceae). Lat.Am.J.Pharm.33,245–250.
Foster,A.S.,1949.PracticalPlantAnatomy,2nded.D.VanNostrand,Princeton. Franceschi,V.R.,Nakata,P.A.,2005.Calciumoxalateinplants:formationand
func-tion.Annu.Rev.PlantBiol.56,41–71.
Fuchs,C.H.,1963.FuchsinstainingwithNaOHclearingforlignifiedelementsof wholeplantsorplantsorgans.StainTechnol.38,141–144.
Gales,R.C.,Toma,C.,2007.Researchesregardingthemorphology,structureand dis-tributionofvegetativeorgansofsomeEuphorbiaspeciesfromRomania’sflora. Biol.Veg.1,40–45.
Gaucher,L.,1902.RecherchesanatomiquessurlesEuphorbiacées.Ann.Sci.Nat.Bot. 15,161–309.
Hagel,J.M.,Yeung,E.C.,Facchini,P.J.,2008.Gotmilk?Thesecretlifeoflaticifers. TrendsPlantSci.13,631–639.
Hajdu,Z.,Hohmann,J.,2012.Anethnopharmacologicalsurveyofthetraditional medicineutilizedinthecommunityofPorvenir,BajoParaguáIndian Reserva-tion,Bolivia.J.Ethnopharmacol.139,838–857.
He, H., Bleby, T.M., Veneklaas, E.J., Lambers, H., Kuo, J., 2012. Morpholo-gies and elemental compositions of calcium crystals in phyllodes and branchletsofAcaciarobeorum(Leguminosae:Mimosoideae).Ann.Bot.109, 887–896.
Johansen,D.A.,1940.PlantMicrotechnique.McGrawHillBook,NewYork. Konno,K.,2011.Plantlatexandotherexudatesasplantdefensesystems:rolesof
variousdefensechemicalsandproteinscontainedtherein.Phytochemistry72, 1510–1530.
Lattanzio,V.,Lattanzio,V.M.T.,Cardinali,A.,2006.Roleofphenolicsintheresistance mechanismsofplantsagainstfungalpathogensandinsects.In:Imperato,F. (org),Phytochemistry:AdvancesinResearch.ResearchSignposts,Trivandrum, pp.23–67.
Luz,L.E.C.,Paludo,K.S.,Santos,V.L.P.,Franco,C.R.C.,Klein,T.,Silva,R.Z.,Beltrame, F.L.,Budel,J.M.,2015.Cytotoxicityoflatexandpharmacobotanicalstudyof leavesandstemofEuphorbiaumbellata(Janaúba).Rev.Bras.Farmacogn.25, 344–352.
Meric,C.,2009.CalciumoxalatecrystalsinsomespeciesofthetribeInuleae (Aster-aceae).ActaBiol.Crac.Ser.Bot.51,105–110.
Metcalfe,C.R.,Chalk,L.,1950.AnatomyoftheDicotyledons.ClarendonPress,Oxford. Nakata,P.A.,2003.Advancesinourunderstandingofcalciumoxalatecrystal
forma-tionandfunctioninplants.PlantSci.164,901–909.
O’Brien,T.P.,Feder,N.,McCully,M.E.,1964.Polychromaticstainingofplantcellwalls bytoluidineblueO.Protoplasma59,368–373.
Oliveira,F.,Akisue,G.,Akisue,M.K.,2005.Farmacognosia.Atheneu,SãoPaulo. Pompert,M.G.,1989.Estudiomorfo-anatomicodedosespeciesdeSapium
Reeve,R.M.,1951.Histochemicaltestsforpolyphenolsinplanttissues.StainTechnol. 26,91–96.
Riederer,M.,Schreiber,L.,2001.Protectingagainstwaterloss:analysisofthebarrier propertiesofplantcuticles.J.Exp.Bot.52,2023–2032.
Roeser,K.R.,1972.DieNadelderSchwarzkiefer-MassenproduktundKunstwerkder Natur.Mikrokosmos61,33–36.
Rudall,P.J.,1987.LaticifersinEuphorbiaceaeaconspectus.Bot.J.Linn.Soc.94, 143–163.
Sass,J.E.,1951.BotanicalMicrotechnique,2nded.IowaStateCollege,Ames. Sátiro,L.N.,Roque,N.,2008.AfamíliaEuphorbiaceaenascaatingasarenosasdo
médiorioSãoFrancisco,BA,Brasil.ActaBot.Bras.22,99–118.
Secco,R.S.,Cordeiro,I.,Senna-Vale,L.,Sales,M.F.,Lima,L.R.,Medeiros,D.,Haiad,B.S., Oliveira,A.S.,Caruzo,M.B.R.,Carneiro-Torres,D.,Bigio,N.C.,2012.Anoverview ofrecenttaxonomicstudiesonEuphorbiaceaes.l.inBrazil.Rodriguésia63, 227–242.
macropadrões.ActaAmazon.44,435–446.
Sobottka,A.M.,Tonial,F.,Sytwala,S.,Melzig,M.,2014.Proteinaseactivityinlatexof threeplantsofthefamilyEuphorbiaceae.Braz.J.Pharm.Sci.50,559–565. Swiech,J.N.D.,Bobek,V.B.,Folquitto,D.G.,Silva,R.Z.,Budel,J.M.,Farago,P.V.,Miguel,
M.D.,Miguel,O.G.,2016.Morpho-anatomyofthevegetativeorgansof
Philoden-dronmeridionale(Araceae).Lat.Am.J.Pharm.35,2142–2148.
ThePlantList,2015.Sapium,http://www.theplantlist.org/browse/A/Euphorbiaceae/ Sapium/(accessedOctober2015).
Upton,R.,Graff,A.,Jolliffe,G.,Länger,R.,Williamson,E.,2011.AmericanHerbal Pharmacopoeia:BotanicalPharmacognosy–MicroscopicCharacterizationof BotanicalMedicines.CRCPress,BocaRaton.
Valle,L.S.,Kaplan,M.A.C.,2000.Sapiumglandulatumcomplex(Euphorbiaceae).An. Acad.Bras.Cienc.72,293–294.