w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Antispasmodic
activity
from
Serjania
caracasana
fractions
and
their
safety
Fabiana
L.
Silva
a,b,
Joelmir
L.V.
da
Silva
c,
Juciléia
M.
Silva
c,
Luiza
S.A.
Marcolin
d,
Viviane
L.A.
Nouailhetas
e,
Massayoshi
Yoshida
f,
Pedro
H.
Vendramini
g,
Marcos
N.
Eberlin
g,
José
M.
Barbosa-Filho
a,
Paulo
R.H.
Moreno
b,∗aLaboratóriodeTecnologiaFarmacêutica,UniversidadeFederaldeParaíba,JoãoPessoa,PB,Brazil bInstitutodeQuímica,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
cDepartamentodeSaúdeII,FaculdadedeFarmácia,UniversidadeNovedeJulho,SãoPaulo,SP,Brazil dDepartamentodeSaúdeIII,FaculdadedeMedicina,UniversidadeNovedeJulho,SãoPaulo,SP,Brazil eDepartamentodeBiofísica,UniversidadeFederaldeSãoPaulo,SãoPaulo,SP,Brazil
fCentrodeBiotecnologiadaAmazônia,Manaus,AM,Brazil
gThoMSonMassSpectrometryLaboratory,InstitutodeQuímica,UniversidadedeCampinas,Campinas,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received30September2016
Accepted20December2016
Availableonline10February2017
Keywords:
Antispasmodicactivity
Compoundsisolation
GC–MS
Hemolyticassay
Ileumrat
Extracttoxicity
a
b
s
t
r
a
c
t
Inapreviousstudy,wereportedtheantispasmodicandgastroprotectiveeffectsoftheSerjania
cara-casana(Jacq.)Willd.,Sapindaceae,extract.Inthepresentstudy,weevaluatedtheLD50,hemolyticand
antispasmodicactivitiesofitsfractionsandcharacterizeditsmajorconstituentsbyisolationandGC–MS. Theanimals showednon-toxicsymptomswithoraldosesup to2000mg/kg, suggestingasafeoral administration.Furthermore,alowhemolyticactivitywasdetectedforthesaponinfraction. Antispas-modicactivityofthefractionswasevaluatedthroughcarbachol-inducedcontractionsinratileum.The hexanefractionwasthemostpotent(IC5068.4±5.9g/ml)followedbythedichloromethanefraction (IC50161.3.4±40.7g/ml).Butanolfractionwasthelesseffective(IC50219.8±60.3g/ml).The phyto-chemicalstudyoftheS.caracasanafractionsaffordedtheisolationoffriedelin,-amyrin,allantoinand quercitrin.ThisisthefirsttimethatthepresenceofallantoinandquercitrinintheSerjaniagenushasbeen reported.AmongtheisolatedcompoundsandthosecharacterizedbyGC–MS,-amyrinand-sitosterol werepresentinthemostactivefractions,hexaneanddichloromethane,andtheymayberelatedtoits antispasmodicactivity.Inaddition,spathulenolwasonlyfoundinthehexanefractionanditspresence mightjustifythehighestantispasmodicactivityobservedforthisfraction.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Serjaniacaracasana(Jacq.)Willd.,Sapindaceae,isusedfor weav-ing baskets and rustic ropes, mostly because it is considered ichthyotoxic.ThisbeliefcomesfromitssimilaritywithS.lethalis, which is knownto contain hemolyticsaponins (Teixeira et al., 1984).Infact,allpreviouspublicationsaboutS.caracasana(Aragão andValle,1973;XavierandMors,1975;CordeiroandValle,1975; Maia-Braggioetal.,1978)usedS.lethalisinstead,asitwaslater confirmed(Teixeiraetal.,1984).
∗ Correspondingauthor.
E-mail:prmoreno@iq.usp.br(P.R.Moreno).
SomeSerjaniaspeciesareusedinfolkmedicinesuchasS.comata
(rheumatism),S.lethalis(kidneypain,anti-inflammatory),S.erecta
(gastricpain,anti-inflammatory),S.marginata(gastricpain)andS. triquetra(diuretic)(ChávezandDelgado,1994;Guarin-Netoetal., 2000;Agraetal.,2008;Péricoetal.,2015).Basedonthesepopular uses,ourgrouphaspreviouslydemonstratedthattheS.caracasana
hydroethanolicextractshowedagastroprotectiveeffectby inhib-itinggastriculcerinductionandaninvitroantispasmodicactivity (Silvaetal.,2012).
Inthisstudy,wefurtherevaluatedtheantispasmodicactivity toidentifythecompoundsresponsibleforthiseffect.Theextract safety was initially evaluated through the determination of its medianlethaldose(LD50)andthesearchforhemolyticsaponins inthebutanolfraction.
http://dx.doi.org/10.1016/j.bjp.2016.12.002
0102-695X/©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://
Materialsandmethods
Chemicalmaterials
StandardsampleofsaponinsofQuillajasaponariawasakind courtesyofProf.Dr.CarmenQueiroga.Thecitratedbovineblood waspurchased attheUSP VeterinaryHospital(USP-Brazil). Sil-ica gel 60 F254 TLC aluminum sheets were purchased from MerckCompany.Allothersreagentsandsolventswereanalytical grade.
Plantmaterial
The aerial parts of Serjania caracasana (Jacq.) Willd., Sapin-daceae,werecollectedatthebaseofPicodoJabre(7◦15′34.27′′ S,37◦23′8.53′′W),Paraiba,Brazilduringfructificationperiod(June, 2009)byDr.JoseanF.Tavares(UFPB).Theplantmaterialwas identi-fiedbyProf.Dr.MariadeFátimaAgra(UFPB).Avoucherspecimen (No.M.F.Agraetal.,6963)wasdepositedintheHerbariumProf. LauroPiresXavier(JPB),atthesameUniversity.
Phytochemicalanalysis
Spectroscopicpropertiesoftheisolatedcompounds
NMR analysis was performed in an Agilent INOVA-500 (500MHz)spectrometer.Highresolutionmassspectrawere per-formedonaMicroTOFLCmassspectrometerfromBrukerDaltonics andIRspectrawasrecordedwithaBomeminstrument.The struc-turesoftheisolatedcompoundsweredeterminedby1Hand13C NMR spectroscopy,using one and two-dimensionaltechniques, togetherwithIRandHRMSdatabycomparisonwithdata previ-ouslypublishedforthesecompounds.
Gaschromatography–massspectrometry(GC–MS)analysis
The hexane and CH2Cl2 fractions had their chemical com-position analyzed by gas chromatography–mass spectrometry (GC/MS),performedusingaShimadzuGCMS-QP2010Ultrasystem, withafusedsilicacapillarycolumncoatedwith5% polyphenyl-siloxane/95% dimethylpolysiloxane (Rxi®-5ms,10m×0.10mm ID×0.10mfilmthickness), electronionizationsystem operat-ingat70eVwithaninterfacetemperatureof 260◦C,scan time of 0.1scans/s and acquisition mass range of 35.0–500.0Da. For theGCanalysis,theinjectiontemperaturewassetat250◦C,the oventemperatureprogram was100◦C for 1min,100–290◦C at 15◦C/min,maintaining290◦Cfor15minwithheliumasacarrier gas(0.42ml/min).Thecompoundidentificationwasperformedby comparingthemassspectrawiththeWileylibraryandliterature data(Adams,2007)togetherwiththeco-injectionofstandards.The relativecompositionwasobtainedfromtheelectronicintegration measurementsusingflameionizationdetection(300◦C)without takingintoaccountrelativeresponsefactors,inthesameconditions describedabove.
Extractpreparation
Thepowderedair-driedaerialpartsofS.caracasana(1916g) were extracted exhaustively with 96% aqueous ethanol solu-tionat roomtemperature.The combinedextractswerefiltered and concentrated under reduced pressure at 40◦C afford-ing a hydroethanolic extract (202.84g; 10.6% extraction yield) (EtOH).
Extractfractionationandcompoundisolation
TheEtOHextract(200g)wassuspendedinMeOH–H2O(70:30, v/v) mixture, and it wassubsequently fractionedwith hexane, CH2Cl2 andBuOH.Eachsolventfractionwasthenevaporatedto drynessunderreducedpressuretogivehexane(31.53g;15.8%),
CH2Cl2 (21.9g;10.96%)andBuOH(44.50g;22.2%)fractions.The CH2Cl2andBuOHfractionswereseparatelysubjectedtoCCon sil-icagelusingstepgradientsofhexane–EtOAcandEtOAc–MeOH. Columnfractionsthatpresentedonlyonemajorcompoundwere resubmitted tothe samechromatographic separation to obtain thepurifiedcompounds.CH2Cl2fractionyieldedfourcompounds (friedelin(1),-amyrin(2),stigmasteroland-sitosterol),while BuOHfractionaffordedonecompound(-sitosterolglucoside).A remainingpartoftheBuOHfractionwassuspendedwith300ml ofMeOH.Thesolutionwasfilteredandinsolublepartreserved. Diethyletherwascarefullyaddedintothesolutionuntilsome pre-cipitationstarted,and thenitwasplacedin thefreezer(−22◦C for24h).Theprecipitateformedwasremovedbyfiltration,and reserved.Thisprocesswasrepeatedtwice.Thepooledprecipitates weresolubilizedwithMeOHandconcentratedunderreduced pres-sureat40◦C,togiveBuOH-1fraction.Theremainingorganicphase wasevaporatedunderreducedpressuretodrynesstogive BuOH-2fraction.TheBuOH-2fractionwassubjectedtoCConsilicagel usingstepgradientsofCHCl3–MeOHandafterfixedsolventsystem (CHCl3–acetone–formicacid,75:16.5:8.5(v/v))(Ikedaetal.,1991) toobtain27fractionscombinedaccordingtotheirTLCprofilesinto fifteenmajorfractions(Fr1–Fr15).FromfractionFr7obtained allan-toin(3)asprecipitateaftersolventdrying.FractionFr8waspurified bypreparativeTLC(CHCl3–MeOH,80:20(v/v))togivequercitrin (4).
Friedelin(1).5.6mg,0.03%yield;amorphouspowder;IRmax (KBr)cm−1:3000,2848,1714;1HNMR(500MHz,CDCl
3):1.16(3H,
s,H-28),1.03(3H,s,H-27),0.99(3H,s,H-26),0.98(3H,s,H-30),0.93 (3H,s,H-29),0.86(3H,slap,H-23),0.85(3H,s,H-25),0.70(3H,s, H-24).13CNMRdatawereconsistentwiththosepreviouslyreported (Akihisaetal.,1992).
-Amyrin(2).169.8mg,1.05% yield;white powder;IRmax (KBr)cm−1:3299,2946,2852,1034;1HNMR(500MHz,CDCl
3): 5.16 (1H, t, J=3.6Hz, H-12), 3.20 (1H, dd, J=11.15, 4.5, H-3), 1.11 (3H, s, H-27), 0.97 (3H, s, H-23), 0.94 (3H, s, H-26), 0.91 (3H, s, H-24), 0.80 (3H, s, H-28), 0.76 (3H, s, H-25). 13C NMR datawereconsistentwiththosepreviouslyreported(Diasetal., 2011).
Stigmasterol. 37.8mg, 0.24% yield; white powder;1H NMR (500MHz;CDCl3):5.33(1H,sl,H-6),5.13(1H,dd,H-23),5.00(1H,
dd,H-22),3.50(2H,m,H-3).13CNMRdatawereconsistentwith thosepreviouslyreported(Kojimaetal.,1990).
-Sitosterol. 21.4mg, 0.13% yield; white powder; 1H NMR (500MHz;CDCl3):5.33(1H,sl,H-6),3.50(2H,m,H-3).13CNMR datawereconsistentwiththosepreviouslyreported(Kojimaetal., 1990).
-Sitosterolglucoside.3.2mg,0.04%yield;amorphous pow-der; 1H NMR (500MHz,Py-d
5): 5.32(1H, sl, H-6), 5.04 (1H, d, H-1′),4.55(1H,map,H-6′),4.28(1H,sap,H-4′),3.99(1H,m, H-3),2.70/2.45(1H,m,H-4),0.98(3H,d,H-21),0.94(3H,sl,H-19), 0.91(3H,sap,H-26),0.87(3H,dap,H-29),0.84(3H,d,H-27),0.64 (3H,s,H-18).13CNMRdatawereconsistentwiththosepreviously reported(Kojimaetal.,1990).
Allantoin(3).6.5mg,0.08%yield;colorlesscrystal;IRmax(KBr) cm−1:3439, 3344,3224,3063,1782,1659; 1HNMR (500MHz, DMSO-d6): 10.51(1H,br s,H-1), 8.03 (1H,s, H-4),6.91(1H, d,
J=13.5Hz,H-3),5.78(2H,s,H-8),5.24(1H,d,J=13.5Hz,H-6).13C NMRdatawereconsistentwiththosepreviouslyreported(Sripathi etal.,2011).EI-HRMS(negative-ionmode)m/z:157.03722[M−H]− (C4H6N4O3requires157.0315).
Toxicologicalassays
Medianlethaldose(LD50)
MaleSwissmice(35–40g)wereseparatedinthreegroupsoffive animalsforeachtreatment(n=5).Animalsfromeach experimen-talandcontrolgroupswerehostedincagesmaintainedatconstant roomtemperature(22±1◦C)andsubjectedtoa12/12hlight/dark cyclewithaccesstofoodandwateradlibitum.Proceduresinvolving animalsandtheircarewereperformedinconformitywithOECD 420(2001),adoptedinourlaboratory,andincompliancewith cur-rentinternationallyacceptedinstructionsforthecareoflaboratory animalsandethicalguidelines.Furthermore,clearancefor conduct-ingthestudywasobtainedfromtheEthicsCommitteeofAnimal UseoftheNovedeJulhoUniversity(approval#AN0003/11).
Inordertodeterminetheoralmedianlethaldose(LD50)ofthe EtOHextract,thistestfollowedthemethoddescribedforMillerand Tainter(1944)withsamemodifications.Thecontrolgroupreceived thevehicle(0.1%Tween20indistillatedwater).Twodoses(1000 and2000mg/kg,inavolumeof1ml/g)weregivenorally,bygavage, totwogroupsofmiceforthedeterminationofLD50.Theanimals wereobservedduringthefirst180minafterthetreatmentandafter 24,48and72hforanytoxicsignsandsymptoms.
Qualitativeassayofhemolyticsaponins
Thequalitativehemolyticactivityofthesaponinswastested withbovinebloodreagentasdescribed bySharmaetal.(2012) withsomemodifications.Briefly,aliquotsof1mlofcitratedbovine bloodwerewashedthreetimeswith9mlofsaline(0.9%;w/vNaCl) followedbycentrifugationat180×gfor5min.Thecell suspen-sionwasfinallypreparedbydilutingthepelletto3%(v/v)insaline solutiontoobtainthebloodreagent.Also,tovisualizethesaponins acomparativesprayreagent(Liebermann–Burchardreagent)was prepared(WagnerandBladt,1996).
Theassaywasperformedwitha10laliquotofsaponinsfrom
Quillajasaponaria(SQ)andBuOHfraction(conc.20mg/ml, solubi-lizedinMeOH–H2O,70:30(v/v))appliedinTLCplateswhichwere elutedwiththesolventsystemCHCl3–MeOH–TFA0.5%(60:40:5 (v/v))to6.5cmfromtheorigin.Aftertheelution,theTLCplates wereairdried.
OneofthedevelopedTLCplateswasimmersedfor20sinaglass dishcontainingthe3%bloodreagentfreshlyprepared.Afterthis time,theTLCplatewasremovedandheldverticallyfor30s,and subsequentlyimmersedinsalinefor30s. Finally,this TLCplate washeldverticallyforcompletedryingandfurthervisualization ofthehemolyticspots.Thehemolyticspotswerecomparedwith thedeveloped TLC sprayed with Liebermann–Burchardreagent (Sharmaetal.,2012).
Quantitativeassayofhemolyticsaponins
Thequantitativehemolyticactivityofthesaponinswas eval-uated with a bovine blood cell suspension by turbidimetry as describedby Xieetal. (2008)withsomemodifications. Briefly, aliquotsof10mlofbloodwerewashedthreetimeswithsaline solu-tionbycentrifugationat180×gfor2min.Thecellsuspensionwas finallypreparedbydilutingthepelletto5%(v/v)insalinesolution. TheSQand BuOHfractionsamplesweresolubilized initially insalineto25mg/ml.Forthe assay,180loffreshly prepared 5% blood cell suspension was mixed with 20l of sample or controlsolution.Thesampleconcentrationrangedfrom820.0to 16.4g/mlforBuOHfraction,andfrom500.0to5g/mlforSQ. Themicroplatewasincubatedfor30minat37◦Candcentrifuged at70×g for10min.Analiquotofeachsupernatant (75l)was transferredtoaflat-bottommicroplateandthefreehemoglobin was measuredat 540nm (Silveira et al., 2011). Saline and SQ (100g/ml)wereconsideredasminimalandmaximalhemolytic controls.Theconcentrationinducing50%ofmaximumhemolysis
(HC50)wascalculated bynon-linerregression (GraphPadPrism 5.01).Eachexperimentincludedtriplicatesforeachconcentration. Theresultsofthequantitativehemolyticactivityarepresentedas mean±SDandtheotherresultsasmean±SEM.
Pharmacologicalassay
Antispasmodicactivityonileumisolatedrat
Ileumstrips wereisolatedfromrat following the methodol-ogydescribed by Walker and Wilson(1979) and suspended in organbaths(5ml)containingmodifiedKrebsphysiological solu-tion,consistingof(mmol/l):NaCl117.0;KCl4.7;NaH2HPO4·H2O 1.2;MgSO4·7H2O1.3;CaCl2·2H2O2.5;NaHCO325.0;glucose11.0; pH7.4(SunandBenishin,1994).Furthermore,theclearancefor conductingthestudywasobtainedfromtheEthicsCommitteeof AnimalUseoftheFederalUniversityofSãoPaulo(approval#CEUA 4195060514/14).
Thehexane,CH2Cl2andBuOHfractionsweredissolvedinitially in0.01%CremophorELanddilutedinMilliQwatertoobtainthe stocksolutionof10mg/mlwhichwasstoredat0◦C.
Thetissuesweremaintainedunder1gtension,bubbled con-tinuouslywithO2at37◦C.Theywereattachedtoforceisometric transducers and connectedto a datasystemAQCD(AVS Proje-tos,Brazil).FollowingcontrolcontractionswithKCl (40mmol/l) or carbachol (1mol/l), and washing with a fresh Krebs solu-tion,tissuestripswereexposedtoconcentrationgradientranging from 500 to 9g/ml of hexane, CH2Cl2 or BuOH fractions for 15min(WalkerandWilson,1979),thenstimulatedagainwiththe previousreferredconcentrationofcarbachol.Theantispasmodic activityofthesampleswasexpressedasmaximum contraction (Emax value) obtained in the presence of the distinct fraction relativetothemaximumcontractionintheirabsence(control). The concentration of fraction that reduces to 50% a maximal responseforanagonist(IC50)valuesweredeterminedfrom indi-vidual concentration–response curves by non-linear regression (Jenkinsonetal.,1995).
Statisticalanalysis
Statisticalsignificancebetweencontrolandexperimentalgroup wasevaluatedusingeitherStudent’st-testorone-wayANOVA fol-lowingDunnett’sMultipleComparisonTest.Datawereconsidered significantwhenp<0.05.
Resultsanddiscussion
Medianlethaldose(LD50)
Inourearlierstudy,weobservedtheS.caracasanaEtOHextract significantly inhibited KCl pre-contracted ileum, indicating an interestinginvitroantispasmodicactivity(Silvaetal.,2012). How-ever,duetothepossibletoxicitytomammalsthatcouldberelated tothepresenceofhemolyticsaponins,asreportedforotherSerjania
A
B
0.6 0.6
Rf Front
0.4
0.2
0.1
Start
1 1
2 2
0.4
0.3
Fig. 1.TLC of BuOH fraction of Serjania caracasana aerial parts (1) and
of rich fraction saponins of Quillaja saponaria (SQ) (2). Solvent system:
chloroform–methanol–trifluoroaceticacidaqueoussolution0.5%(60:40:5,v/v).
Detection:bloodreagent3%(A);Liebermann–Burchardreagent(B).
andhydroethanolicextractfromS.marginata,wheremicetreated withasingleoraldoseof5000mg/kghadnosignsoftoxiceffects inanacutetoxicitystudy(Arrudaetal.,2009;Péricoetal.,2015).
Qualitativeandquantitativeassaysofhemolyticsaponins
DespitethelowacutetoxicityofS.caracasanaEtOHextract,the presenceofhemolyticsaponinsinSerjaniaextractsshouldbe inves-tigated.Thesecompoundsareconsideredharmfulnotonlybecause oftheiracutetoxiceffects, asreportedtothesaponinsisolated fromS.lethalis,serjanosidesA,BandC,thatshowedtoxicacute effectsinratsandmice(Teixeiraetal.,1984).Also,chronic admin-istrationofsaponinsinanimalsisknowntoaffecttheirgrowth oralteringtheirpalatabilityand,consequently,foodconsumption oralteringthedigestionprocessandabsorption(Oleszek,1996). TheTLCchromatogramoftheS.caracasanaBuOHfractionsprayed withLiebermann–Burchardreagent(Fig.1B)showedthepresence ofthreemainpurplezones,characteristicofsaponins,atRf’s0.3,
0.4and 0.6, withthelatterasthemajor component.The com-parisonofthisTLCprofilewiththatobtainedaftersprayingwith theBloodreagent(Fig.1A)indicatedthattheobservedsaponins werenotabletocausehemolysisintheTLCassayinthe concen-trationtested.Incontrast,thepositivecontrol(SQ)showedsome whitespots(Rf=0.1and0.2)againstapinkbackground(Fig.1A),
characteristic of hemolysis. However, SQ also presented two othercompounds thatwerenon-hemolytic compounds(Rf=0.4
and 0.6), typically saponins for Liebermann–Burchard reagent (Fig.1B).
Similarly,inthequantitativehemolyticactivity,theBuOH frac-tion exhibited a low hemolysis rate(∼2%) until themaximum concentrationtested(820g/ml)whencomparedwiththepositive control (SQ) (100% at 100g/ml) (Fig. 2). Once at the maxi-mumconcentrationtestedthehemolysisrateswereunder50%of hemolyticactivity,theBuOH fractionHC50valuewasestimated as>1000g/ml.Thepositivecontrol(SQ)showedhighhemolytic activity (HC50=24.03±1.03g/ml) under the same conditions. Thus,differentthanwouldbeexpectedforanichthyotoxicspecies, ourresultsindicatedthatS.caracasanasaponinscouldbe consid-eredasnon-hemolytic.
Antispasmodicactivityonileumisolatedrat
As S. caracasana demonstrated low toxicity and almost no hemolyticeffect, we decided toinvestigate which extract frac-tionwouldberesponsiblefortheantispasmodicactivitypreviously reported(Silvaetal.,2012).
TheantispasmodicactivitywasanalyzedfortheS.caracasana
hexane,CH2Cl2andBuOHfractions.Fig.3(A)–(C)showsthe over-allinhibitoryeffectofthesefractionsonratileumcontractions. Thehexane fractionsignificantly inhibitedthemaximumileum contractionsattheconcentrationsof27,81,243and500g/ml (Fig.3A),presentingtherespectiveamplitudedecreasevaluesof 66.1±7.1,47.5±3.8,24.5±2.4and23.5±7.3%(Fig.3A),whilefor theCH2Cl2fractionasignificantinhibitionstartedatthe contrac-tionsof81,243and 500g/ml,withEmax valuesof62.7±12.6, 47.7±7.9and 20.2±3.7%respectively (Fig.3B).In contrast,the BuOHfractionwasonlyabletosignificantlyreducetheintensity ofthecontractionsat243and500g/mlwithrespectiveEmaxof 44.9±13.1and25.8±5.5%(Fig.3C).
Theseresultsindicatedthattheantispasmodicactivityis dis-tributed in all S. caracasana fractions. However, the n-hexane fractionwasmorepotentthanthecrudeEtOHextract,thatwas 46%attheconcentrationof81g/ml(Silvaetal.,2012).TheCH2Cl2 fractionwaslessactiveshowingsimilarresultstothoseobtained withthecrudeextract,andtheBuOHfractionshowedevenless activitythantheextract.Thus,allthefractionswereinterestingto searchfornaturalantispasmodiccompounds.
Phytochemicalanalysis
Inordertodeterminethepossiblecompoundsresponsiblefor theantispasmodicactivity,CH2Cl2andBuOHfractionswere sub-mittedtocolumnchromatography.Fromthesefractions,wecould
100
A
B
75
50
25
0
0 100 200
[SQ] µg/ml
Hemolysis
, %
Hemolysis
, %
300
0
0 200 400 600
[BuOH] µg/ml
800 1000 2
4 6 8 10
100
A
B
C
7550
25
0
CCh 9 27 81 243 500
[Hexane] µg/ml
Contr
actions
, %
100
75
50
25
0
CCh 9 27 81 243 500
[CH2CI2] µg/ml
Contr
actions
, %
100
75
50
25
0
CCh 9 27 81 243 500
[BuOH] µg/ml
∗∗∗
∗∗∗ ∗∗∗ ∗∗∗
∗∗∗ ∗∗∗ ∗∗∗ ∗∗
∗∗
Contr
actions
, %
Fig.3. Effectofhexane(A),CH2Cl2 (B)orBuOH(C)fractionsofSerjania
cara-casanaaerialpartsonpre-contractedileumbycarbachol1M(n=3).One-way
ANOVAfollowedbyDunnett’sMultipleComparisonTest:**p<0.01;***p<0.001
(control×fraction).
isolateandcharacterizesevencomponents,twooleanane triter-penes:friedelin(1)and-amyrin(2),threesteroids:stigmasterol, -sitosteroland-sitosterolglucoside,oneuratederivative: allan-toin(3)andoneflavonol:quercitrin(4).
Table1
CompoundscharacterizedinthehexaneandCH2Cl2fractionsofSerjaniacaracasana
aerialparts.
Hexanefraction CH2Cl2fraction
Compound RT(min) % RT(min) %
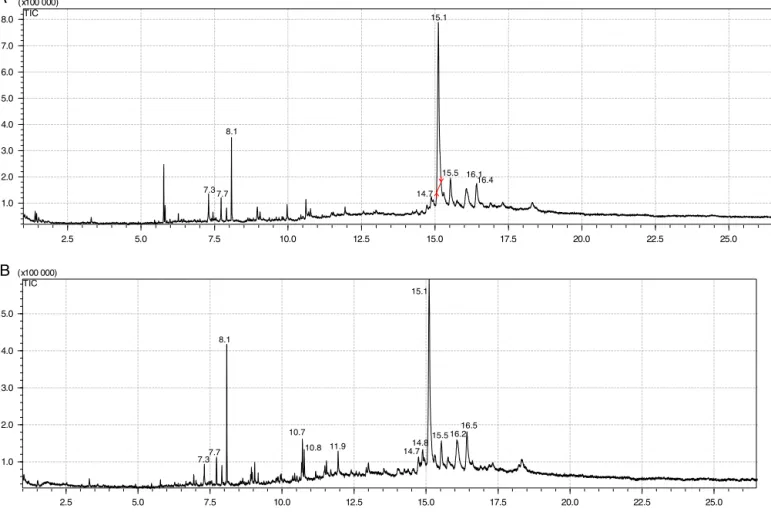
Spathulenol(5) 5.8 4.2 – –
6,10,14-Trimethyl-2-pentadecanone 7.3 1.7 7.3 1.8
Methylpalmitate 7.7 1.7 7.7 2.7
Ethylpalmitate 8.1 6.2 8.1 8.6
-Sitosterol 14.7 1.2 14.7 2.1
-Amyrin(2) 15.1 60.0 15.1 51.0
Total 75.0 66.2
O
H H H
1
HO
H
2 H
H
H N
N H O
N H
O O
NH2
3
O HO
OH
O
O
OH
OH
O HO
HO
OH
4
Additionally,thetwomostactivefractions,hexaneandCH2Cl2, hadtheirchemicalcompositioncomparedbyGC–MS,indicatinga similarchemicalcompositionforthesefractions(Fig.4).Themass spectraobtainedallowedtocharacterizesixcompounds(Table1) representing79.8%and66.6%ofthetotalofcompoundsdetectedin theGC–MS.Thetwofractionsshowedthesamemajorcomponents, -amyrin(2)(60.0%and51.0%)andethylpalmitate(6.2%and8.6%), respectivelyforhexaneandCH2Cl2fractions.Additionally,inthe hexanefractionwasfoundspathulenol(5)inarepresentative quan-tity(4.2%).Thissesquiterpeneisacommonessentialoilcomponent inseveralplantspecies,ithasshownanantispasmodicactivityin uterusringscontractionmodelinducedbyKCl(Perez-Hernandez etal.,2008).Thepresenceofspathulenolexclusivelyinthehexane fractionmightjustifythehighestantispasmodicactivityobserved incomparisontotheotherfractions.
HO H
H
H H
5
In a recent study, Coutinho et al. (2015) reported the seed fatty-acidcompositionsforsixteenSapindaceaespecies.Serjania
species,includingS.caracasana,presentedhighlevelseicosenoic acidand other palmitic acidesters. In ourstudy, palmitic acid methylandethylesterswerefoundinbothhexane andCH2Cl2 fractions.Friedelin(1)and-amyrin(2),togetherwithother triter-penes,aretypicallyisolatedwithinthegenusSerjania,suchasthe presenceof1and2reportedinS.salzmanniana(Barbosa-Filhoetal., 1988).In thisstudy,wedetectedforthefirst timeina Serjania
2.5 5.0 7.5 10.0 12.5 15.0 17.5 20.0 22.5 25.0 1.0
2.0 3.0 4.0 5.0 6.0 7.0 8.0
(x100 000) TIC
2.5 5.0 7.5 10.0 12.5 15.0 17.5 20.0 22.5 25.0 1.0
2.0 3.0 4.0 5.0
(x100 000) TIC
8.1
7.3 7.7
10.7
10.8 11.9
15.1
14.714.8 16.216.5 15.5 15.1
7.3 7.7 8.1
14.7 15.5 16.1
16.4
A
B
Fig.4.GC–MSchromatogramofhexane(A)andCH2Cl2(B)fractionsofSerjaniacaracasanaaerialparts.
Accordingtoourresults,S.caracasanasaponinsandquercitrin arenotthemainmetabolitesresponsiblefortheantispasmodic effect observed, but they may contribute to the overall effect observed in the crude extract. In similar studies, it has been observedthatthefractionsenrichedwithsaponinsandflavonol derivatives,includingquercitrin,showedsignificantantispasmodic activityintheacetylcholinemodel(Truteetal.,1997).
In conclusion,ourfindings demonstrated thatalthough ofS. caracasanaisconsideredanichthyotoxicspecies,thisproperty,if present,cannotberelatedtothepresenceofhemolyticsaponins. Thetoxicityformammalspecieswasnotconfirmedbytheacute toxicitytest,requiringcomplimentarystudiesonthesubject.In addition,future studiesshouldbecarried outwiththeisolated compoundstoverifytheirspasmolyticactivity.
Authors’contributions
FLS(Ph.D.student)contributedbyconductingthe phytochem-icallaboratorywork,thehemolyticevaluation anddraftingthe paper.JLVScontributedbyconductingthepharmacologicalassays, teachingJMSandLSAMintheconductingofthepharmacological assaysandbycriticalreadingofthemanuscript.VLANsupervised thepharmacologicalworkandcontributed tocritical readingof themanuscript.MYcontributedwiththecharacterizingofmany isolatedsecondarymetabolites.PHVcontributeddeterminingthe EI-HRMSofallantoin.MNEsupervisedthelaboratoryworkand con-tributedtocriticalreadingofthemanuscript.JMBFdesignedthe phytochemicalworkandcontributedguidingandsupportingthe plantcollectionandthelaboratorywork.PRHMcontributed sup-portinganddesigningthephytochemicalworkandthehemolytic
assays,supervisedthelaboratoryworkandcontributed to criti-calreadingofthemanuscript.Alltheauthorshavereadthefinal manuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Ethicalresponsibilities
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftheresponsibleClinicalResearchEthicsCommitteeandin accordancewiththoseoftheWorldMedicalAssociationandthe Helsinki Declaration.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearsinthisarticle.
Righttoprivacyandinformedconsent.Theauthorsdeclarethat nopatientdataappearsinthisarticle.
Acknowledgments
TheauthorsaregratefultoCNPq/RENORBIOandCAPES/Brazil forfinancialsupportandresearchfellowships.
References
Adams,R.P.,2007.IdentificationofEssentialOilComponentsbyGas
Agra,M.F.,Silva,K.N.,Basílio,I.J.L.D.,Freitas,P.F.,Barbosa-Filho,J.M.,2008.Survey ofmedicinalplantsusedintheregionNortheastofBrazil.Rev.Bras.Farmacogn. 18,472–508.
Akihisa,T., Yamamoto,K.,Tamura,T.,Kimura,Y.,Iida,T.,Nambara,T.,Chang,
F.C.,1992.TriterpenoidketonesfromLingnaniachungiiMcClure:arborinone,
friedelinandglutinone.Chem.Pharm.Bull.40,789–791.
Aragão,J.A.,Valle,J.R.,1973.IctiotixicidadedetimbósdosgênerosSerjania,Derrise
Tephrosia.CiênciaeCultura25,643.
Arruda, A.P.C.C.B.N.,Coelho, R.G., Honda, N.K., Ferrazoli, C.,Pott, A.,
Hiruma-Lima,C.A.,2009.GastroprotectiveeffectofSerjaniaerectaRadlk(Sapindaceae):
involvementofsensoryneurons,endogenousnonproteinsulfhydryls,andnitric oxide.J.Med.Food.12,1411–1415.
Barbosa-Filho,J.M.,Araujo,V.T.,Bhattacharyya,J.,1988.Chemicalconstituentsof
Serjaniasalzmanniana.Fitoterapia59,430–431.
Bulgheroni,A.,Kinsner-Ovaskainen,A.,Hoffmann,S.,Hartung,T.,Prieto,P.,2009.
Estimationofacuteoraltoxicityusingthenoobservedadverseeffectlevel (NO-AEL)from28dayrepeateddosetoxicitystudiesinrat.Regul.Toxicol.Pharmacol. 53,16–19.
Chávez,M.I.,Delgado,G.,1994.Isolationandrelaysysthesisof11˛-hydroperoxy
diacetylhederagenin,anoveltriterpenoidderivativefromSerjaniatriquetra
(Sapindaceae).Biogeneticimplications.Tetrahedron50,3869–3878.
Cordeiro,E.A.,Valle,J.R.,1975.Ictiotixicidadecomparadadarotenonaedo
ser-janosídeo.Cien.Cultura27,561.
Coutinho,D.J.G.,Barbosa,M.O.,Silva,R.M.,Silva,S.I.,Oliveira,A.F.M.,2015.Fatty-acid
compositionofseedsandchemotaxonomicevaluationofsixteenSapindaceae species.Chem.Biodivers.12,1271–1280.
Dias,M.O.,Hamerski,L.,Pinto,A.C.,2011.Separac¸ãosemipreparativade␣e
-amirinaporcromatografialíquidadealtaeficiência.Quim.Nova34,S1–S6.
Guarin-Neto,G.,Santana,S.R.,Silva,J.V.B.,2000.Notasetnobotânicasdeespéciesde
SapindaceaeJussieu.Acta.Bot.Bras.14,327–334.
Ikeda,Y.,Sugiura,Y.M.,Fukaya,C.,Yokoyama,K.,Hashimoto,Y.,Kawanishi,K.,
Moriyasu,M.,1991.PeriandradulcinsA,BandC:phosphodiesteraseinhibitors
fromPeriandradulcisMart.Chem.Pharm.Bull.39,566–571.
Jenkinson,D.H.,Barnard,E.A.,Hoyer,D.,Humphrey,P.P.A.,Leff,P.,Shankley,N.P.,
1995.Internacionalunionofpharmacologycommitteeonreceptor
nomencla-tureanddrugclassification.IX.Recommendationsontermsandsymbolsin quantitativepharmacology.Pharm.Rev.47,255–266.
Kojima,H.,Sato,N.,Hatano,A.,Ogura,H.,1990.SterolglucosidesfromPrunella
vulgaris.Phytochemistry29,2351–2355.
Maia-Braggio,M.,Lapa,A.J.,Valle,J.R.,1978.Mecanismodaac¸ãotóxicadaSerjania
caracasana(Jacq.)Willd.CiênciaeCultura30,455.
Miller,L.C.,Tainter,M.L.,1944.EstimationoftheLD50anditserrorbymeansof
logarithmicprobitgraphpaper.Proc.Soc.Exp.Biol.Med.57,261–264.
Oleszek,W.,1996.AlfafaSaponins:Structure,BiologicalActivity,and
Chemotaxon-omy.InWaller,G.R.,Yamasaki,K.(org.)SaponinsUsedinFoodandAgriculture. PlenumPress,NewYork,pp.155–170.
Perez-Hernandez,N., Ponce-Monter,H.,Medina, J.A.,Joseph-Nathan, P., 2008.
SpasmolyticeffectofconstituentsfromLepechiniacaulescensonratuterus.J. Ethnopharmacol.115,30–35.
Périco,L.L.,Heredia-Vieira,S.C.,Beserra,F.P.,dosSantos,R.C.,Weiss,M.B.,Resende,
F.A.,Ramos,M.A.S.,Bonifácio,B.V.,Bauab,T.M.,Varanda,E.A.,Gobbi,J.I.F.,da
Rocha,L.R.M.,Vilegas,W.,Hiruma-Lima,C.A.,2015.Doesthegastroprotective
actionofamedicinalplantensurehealingeffects?Anintegrativestudyofthe biologicaleffectsofSerjaniamarginataCasar.(Sapindaceae)inrats.J. Ethnophar-macol.172,312–324.
Sharma, O.P., Kumar, N., Singh, B., Bhat, T.K., 2012. An improved method
for thin layer chromatographic analysis of saponins. Food Chem. 132, 671–674.
Shi,S.Y.,Zhang,Y.P.,Zhou,H.H.,Huang,K.L.,Jiang,X.Y.,2010.Screeningand
identifi-cationofradicalscavengersfromNeo-Taraxacumsiphonanthumbyonlinerapid screeningmethodandnuclearmagneticresonanceexperiments.J. Immunoas-sayImmunochem.31,233–249.
Silva,J.L.V.,Carvalho,V.S.,Silva,F.L.,Barbosa-Filho,J.M.,Rigoni,V.L.S.,
Nouail-hetas,V.L.A.,2012.GastrointestinalpropertyofSerjaniacaracasana(Jacq.)Willd.
(Sapindaceae)onrats.PharmacologyonlineS1,22–26.
Silveira,F.,Rossi,S.,Fernández,C.,Gosmann,G.,Schenkel,E.,Ferreira,F.,2011.
Alum-typeadjuvanteffectofnon-haemolyticsaponinspurifiedfromIlexandPassiflora
spp.Phytother.Res.25,1783–1788.
Sripathi,S.K.,Gopal,P.,Lalitha,P.,2011.AllantoinfromtheleavesofPisoniagrandis
R.Br.Int.J.Pharm.LifeSci.2,815–817.
Sun,Y.D.,Benishin,C.G.,1994.K+channelopenersrelaxlongitudinalmuscleof
guinea-pigileum.Eur.J.Pharmacol.271,453–459.
Teixeira,J.R.M.,Lapa,A.J.,Souccar,C.,Valle,J.R.,1984.Timbós:ichthyotoxicplants
usedbyBrazilianIndians.J.Ethnopharmacol.10,311–318.
TheOrganisationofEconomicCo-operationDevelopment(OECD),2001.OECD
GuidelineforTESTINGofchemicals:420AcuteOralToxicology–FixedDose Procedure.TheOrganisationofEconomicCo-operationDevelopment(OECD), pp.1–14.
Trute, A., Gross,J., Mutschler, E., Nahrstedt,A., 1997. Invitro antispasmodic
compoundsofthedry extractobtained fromHederahelix. PlantaMed.63, 125–129.
Wagner,H.,Bladt,S.,1996.PlantDrugAnalysis:AThinLayerChromatographyAtlas,
2ndedition.Springer-Verlag,Heidelberg,Germany,pp.305–327.
Walker, R., Wilson, K.A., 1979. Prostaglandins and the contractile action of
bradykininonthelongitudinalmuscleofratisolatedileum.Br.J.Pharmacol. 67,527–533.
Xavier,H.S.,Mors,W.B.,1975.AssaponinastóxicasdeSerjaniacaracasana.Cien.
Cultura27,179.
Xie,Y.,Ye,Y.-P.,Sun,H.-X.,Li,D.,2008.Contributionoftheglycidicmoietiestothe