w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
article
Anti-inflammatory
activity
and
chemical
analysis
of
extracts
from
Trifolium
riograndense
Graziele
P.R.
Pedrazza
a,
Cláudia
B.
Morais
a,
Greice
R.
Dettenborn
a,
Paula
C.
Ceolato
a,
Miriam
A.
Apel
a,
Elfrides
E.S.
Schapoval
a,
Miguel
Dall’Agnol
b,
José
A.S.
Zuanazzi
a,∗aProgramadePós-graduac¸ãoemCiênciasFarmacêuticas,UniversidadeFederaldoRioGrandedoSul,PortoAlegre,RS,Brazil
bProgramadePós-graduac¸ãoemZootecnia,UniversidadeFederaldoRioGrandedoSul,PortoAlegre,RS,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received15June2016 Accepted30November2016 Availableonline28January2017
Keywords: HPLC Isoflavones Leguminosae Neutrophilchemotaxis Ratpawedema
a
b
s
t
r
a
c
t
Aimingtoinvestigatenewtherapeuticagentswithfewersideeffects,thenumberofstudiesaboutnatural
productshasincreased.Phenoliccompoundscompriseawell-studiedclassofabundantplant-derived
compounds,whoseanti-inflammatoryactivityhasbeendescribed.Isoflavonesarephenoliccompounds
thatoccur mainlyintheLeguminosaefamily,andcanbefoundinmanyspecies,suchasTrifolium
riograndenseBurkart,Leguminosae(clover).InthisstudyanHPLCmethodwasusedtodetermineand
quantifyfourisoflavones(genistein,daidzein,formononetin,andbiochaninA)inhydrolyzedleaf,flower,
stolon,androotextractsofT.riograndense.Invivoanti-inflammatoryactivitywasinvestigatedusingthe
ratpawedemamethodandinvitrochemotaxismodelwithadryextractfromtheleaves,whichhadthe
highestamountofisoflavones.Themajorisoflavonefoundinallpartsoftheplantwasformononetin.
Thechemotaxisassayrevealedthatthedifferentconcentrations(0.2–50g/ml)ofthedryextract
sig-nificantlyinhibitedneutrophilmigrationinaconcentration-dependentmanner(morethan90%).Inthe
ratpawedematest,oraladministrationofcloverextract100mg/kgwasabletosignificantlyinhibit
theedemaformationinducedbycarrageenan.Inconclusion,chemicalanalysesshowedthatTrifolium
riograndenseisaplantrichinisoflavonesandanewinterestingoptionasisoflavonesource.Theresultsof
thebiologicalteststakentogethershowthattheextractofT.riograndensehasanti-inflammatoryeffect
inrodents.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen
accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Severalclassesofsecondarymetabolitesareknowntohave anti-inflammatoryactivities,suchasterpenes,alkaloidsandphenolic compounds.Amongthese,flavonoidsarethecompoundswiththe widestvarietyofactivitiesreported,beingtheanti-inflammatory property attributed to the ability of the compounds to inhibit bothcyclooxygenaseandthe5-lipoxygenasemetabolicpathway ofarachidonicacid(Winekenstaddeetal.,2015;Honmoreetal., 2016).Furthermore,studieshaveshownthatflavonoidsareable toincreasecapillarypermeabilityandexertaninhibitoryeffecton proteinexudationandleukocytemigration(Liuetal.,2016).
Isoflavones,a class of phytoestrogens,are plantmetabolites structurallysimilartothesteroidalestrogen17--estradiol.These compounds have become the object of widespread attention
∗ Correspondingauthor.
E-mail:zuanazzi@ufrgs.br(J.A.Zuanazzi).
as potentialtherapeutic agents, particularlyin women’s health contexts.Inadditiontotheirestrogenicactivity,thesecompounds havebeenassociatedwithpreventionofbreastandprostatecancer as well as cardiovascular disease and inflammatory conditions (Cavendishetal.,2015;Jietal.,2016;Sahpazetal.,2016;Zhang etal.,2016).
TheTrifoliumtaxonisoneofthemostimportantgeneraofthe Leguminosaefamily,duetoitsagriculturalvalueandthe consider-ablenumberofconstituentspecies(about230)(Gilletetal.,2001). Moststudiescarriedouttocharacterizeisoflavonelevelsand quan-tify biologicalactivitieswereperformed withTrifoliumpratense
L.(redclover).Thespeciescontainsrelatedisoflavoneglycosides, mainlytheaglyconesbiochaninA(1)andformononetin(2),besides smaller amounts of daidzein (3) and genistein (4) glycosides (Lemezieneetal.,2015;Tavaetal.,2015).However,theliterature doesnot citestudiesonTrifoliumriograndense Burkart, Legumi-nosae.ThiscloverspeciesnativetosouthernBrazil,especiallythe northernregionofRioGrandedoSulstateisanherbaceous peren-nialplantthatgrowsto50cminheight.Theleavesaretrifoliate
http://dx.doi.org/10.1016/j.bjp.2016.11.004
(withthree leaflets),and theflowersaredarkpink.Thisclover bloomsinspring,anditiscoldresistant.Trifoliumriograndenseis especiallyinterestingtoforageplantbreedersbecauseofits toler-ancetoacidicandaluminum-richsoils(Burkart,1987),acondition quitecommoninthisregion.
HO O
O
R2
R1
R1=OH; R2=OCH3
R1=H; R2=OCH3
R1=R2=OH
R1=H; R2=OH
Theaimofthisworkwastoquantifytheisoflavoneaglycones daidzein, formononetin, genistein, and biochaninA in different organsofTrifoliumriograndense(leaf,stolon,flower,androot)using HighPerformanceLiquidChromatography(HPLC),andtoevaluate theinvivoandinvitroanti-inflammatoryactivityofadryextract preparedwithT.riograndenseleaves.
Materialsandmethods
Plantmaterial
TrifoliumriograndenseBurkart,Leguminosae,wascollected dur-ingitsfloweringstage,inNovember2007,inseveralcitiesinnorth RioGrandedoSulstate,Brazil.Theplantmaterialwasidentified bythebotanistDra.SilviaT.S.Miottoandavoucherspecimenwas depositedattheHerbariumintheICNHerbarium,UFRGS,Porto Alegre,Brazil(number157822).Theleaves,stolons,flowers,and rootsofthegatheredplantsweresortedanddriedinanovenat 100◦Cfor1h.Next,theplantmaterialwasgroundusingamortar
andpestle.
Chemicalsandreagents
Daidzein,genistein,carrageenanandindomethacinwere pur-chasedfromSigma–Aldrich;formononetinandbiochaninAwere purchasedfromFluka.Acetonitrile(HPLCgrade)wasobtainedfrom Merck;HCl, methanol,dichloromethane,and ethanolwere pur-chasedfromVetec;andtrifluoroaceticacid(analyticalgrade)was obtainedfromNuclear.
PreparationoftheextractsforHPLCanalysis
Eachsamplewaspreparedandanalyzedintriplicate.Initially, 10mgofpulverizedplantwasextractedwith4mlof6MHCland incubatedat100◦Cfor15mininwaterbathundermagnetic
stir-ring.Aftercooling,theresidue wasfiltratedandextracted with 15mlofdichloromethane(threetimes).Theextractwas concen-tratedunderreducedpressure,dissolvedin10mlofmethanol.The extractwasfiltratedthrougha0.45mmembranebeforeinjection intheHPLCsystem(Ramosetal.,2008).
HPLCanalysisofextractsforisoflavonecontent
TheHPLCanalyseswereperformedaccordingtoRamosetal. (2008),ona WatersAlliance2695chromatographwitha diode arraydetector(UV/VISWaters2487).Thesystemwasequipped withaC18reverse-phasecolumn(Nova-Pak,4m,3.9×150mm) withguard-columnandoperatedatroomtemperature.Elutionof isoflavoneswasperformedusingalineargradientsystem,andthe
mobilephase consistedof acetonitrile:water:trifluoroaceticacid (20:80:0.01(v/v/v))(A)andacetonitrile:trifluoroceticacid(100:0.1 (v/v))(B).The gradientprofilewas: 0–10min from0to40% B, 10–11min40%B,11–12minfrom40to100%B.Attheendofeach run,100%Awasusedfor6mintorestoretheinitialconditions.The flow-ratewas0.7ml/min.Thedetectionwavelengthwas260nm.
Theidentificationofisoflavoneswasperformedbycomparing theUVprofilesandretentiontimeswithchemicalreference sub-stances.Standardcurvesweregeneratedforthefourisoflavones (daidzein, formononetin, genistein, and biochanin A). The area under the curve for each isoflavone of the extract was deter-mined,andtheseareaswereusedtocalculatethepercentweight of isoflavones in the samples, based on standard curve, linear regression,andamountinjectedinthecolumn.RelativeStandard Seviations(RSD)forareavaluesfromtriplicateinjectionswere cal-culated as:RSD=[(mean−standard deviation)/mean]×100, and thesamples’RSDhadtobe<5%tobeconsideredvaliddata.
Dryextractpreparation
The dry extract was obtained by soaking the leaves of T. riograndensedriedandcrushedwith40%ethanolatroom tempera-turethreetimes,eachtimeforthreedays.Theethanolextractwas partitionedwithdichloromethane.Thesolventwasremovedunder reducedpressureandtheresultingconcentratedextractwas dis-solvedinwaterandsubjectedtolyophilizationtoproducethedry extract.
Animals
Wistarrats(180–220g)wereobtainedfromtheBreeding Lab-oratory,UFRGS,Brazil.Theanimalswerehousedfourpercagein atemperaturecontrolledroomwithfreeaccesstofoodandwater. ThisstudywasapprovedbytheEthicalCommitteefrom Universi-dadeFederaldoRioGrandedoSul(protocolnumber:2007981).
Anti-inflammatoryactivities
Chemotacticmigration
Chemotacticmigrationwasmeasuredaccordingtothemethod describedbySuyenagaetal.(2011).Atotalofsevenratswereused in this assay.For obtainingrat polymorphonuclear neutrophils, 20ml of sterile 1%glycogen (w/v) were injectedinto the peri-toneum of one Wistar rat and 4hlater, theanimal waskilled bydecapitationandtheleukocytescollected.T.riograndensedry extractwasdissolvedinratleukocytessolutiontothe concentra-tionsof100, 50, 25,10, 5,1,0.5, and0.2g/ml, andincubated at37◦Cfor30min.Plasmacollectedfromsixratswasincubated
at37◦Cfor30minwith65g/mlofLPS(lipopolysaccharidefrom
Escherichiacoli)anddilutedinHanksbuffertoa20%solution(v/v). ThereferencedrugsbiochaninA(1),formononetin(2),daidzein(3), andgenistein(4)(10g/ml)werealsodissolvedinHanksbuffer.
Theleukocyte/sampleswereaddedintheupperwellsofthe chamber,separatedbyan8.0mnitrocellulosefilter(Millipore, USA)fromthechemotacticstimulant(LPS)presentinthebottom compartment.Thechamberwaskeptat37◦Cfor1hand,afterthat,
theleucocytesmigrationthroughthefilterwasmeasuredbyusing anopticalmicroscope.Thedistancefromthetopofthefiltertothe farthestplaneoffocuscontainingtwocellsallowedtheevaluation ofleukocytemigration.Measurementsweretakenfromfivefields acrosseachoneofduplicatefiltersandtheresultsexpressedas mean±standarderrorofthemean(SEM).
Carrageenan-inducedpawedemainrats
1.40
1.20
1.00
0.80
0.60
0.40
0.20
0.00
2.00
220.00 220.00 220.00 220.00
380.00 380.00
380.00 380.00
nm 5636
1 - Daidzein 2 - Genistein 3 - Formononetin 4 - Biochanin
13.04 261.7
9.59 299.8 247.4 8.07 256.9
5.64
384.7 365.4 299.8 247.4 209.6
A nm
8077
nm 9594
nm 13 041 4.00 6.00
5636
8077
9594
13 041
8.00 10.00
Minutes 4 3
2
1
AU
12.00 14.00 16.00 18.00 20.00
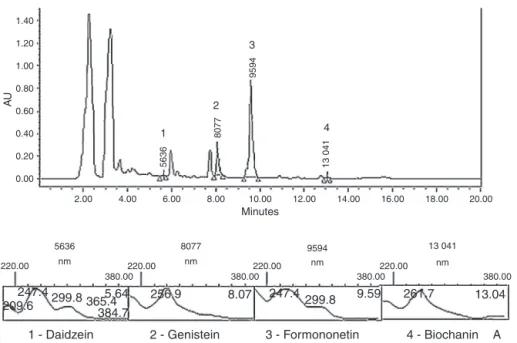
Fig.1. ChromatogramandUVspectra(260nm)oftheisoflavonesdaidzein,genistein,formononetin,andbiochaninAfoundinTrifoliumriograndense.
andtestgroupoffiveanimalseach.T.riograndensedryextractwas resuspendedinsalineandadministeredorally1hbefore subplan-tarinjectionof carrageenan(0.1mlof asuspensionat5mg/ml) usinga singledoseof100mg/kgbodyweightforeachgroupof samples(n=5).The controlgroup receivedequivalentvolumes ofthevehicle.Theactivitywascompared withtheeffectofthe positivecontrolindomethacin(99%purity;Sigma)administration (10mg/kginsaline,p.o.).
MaleWistarratswereanaesthetizedwithsodium pentobarbi-tal(40mg/kg,i.p.)andinjectedsubplantarlyintooneofthehind pawswith0.1mlof0.5%-carrageenantypeIVsolutioninisotonic saline(SigmachemicalCo.,St.Louis,MO).Thecontralateralpaw wasinjectedwith0.1mlsalinesolutionandusedascontrol.Edema wasmeasuredusingadigitalplethysmometerUgoBasile(model 7140,Italy)at1,2,3and4haftercarrageenaninjection.Edema volumewasexpressedforeachanimalasthepercentagechangein ratpawvolumeaftercarrageenaninjectioncomparedwithplacebo group.
Statisticalanalysis
Resultsareexpressedasthemean±SEMandweretestedfor significanceusingStudent’st-test.Probabilityvalues(p)oflessthan 0.05weretakentoindicatestatisticalsignificance.
Resultsanddiscussion
InthisHPLCsystem,daidzein(3),genistein(4),formononetin (2) and biochanin A (1) wereeluted with thefollowing reten-tiontimeranges:5.646,8.077,9.594,and13.041min,respectively (Fig.1).R2valuesfortheleast-squareregressionequationsfittedto thestandardcurveswereasfollows:daidzein(0.9983),genistein (0.9999),formononetin(0.9996),andbiochaninA(0.9997).
Theconcentrationsofthefourinvestigatedisoflavonesin dif-ferentpartsfromT.riograndensearesummarizedinTable1.Total isoflavone concentration was18.30mg/g of dry plant material. In leaves, total isoflavone concentrationwas 7.331mg/g of dry plant. The root presented the lowest isoflavone concentration, 2.806mg/g.Themainisoflavonefoundinallpartsoftheplantwas formononetin(16.683mg/g)followedbybiochaninA(1.207mg/g). Theisoflavone concentration foundin Trifolium riograndense
ishigh, whencomparedwithotherspecies oftheLeguminosae
Table1
Isoflavonecontents(milligrampergramofdryweight;arithmeticmeansofthe analyticaldataaregiven;numberofreplicates:3)indifferentpartsofTrifolium riograndense.
Plantorgan Isoflavones(mg/g)
Daidzein Genistein Formononetin BiochaninA Total
Leaves 0.063 0.167 6.623 0.478 7.331
Flowers 0.059 0.015 3.180 0.188 3.442
Stolons 0.065 0.033 4.348 0.275 4.721
Roots 0.000 0.008 2.532 0.266 2.806
Total 0.187 0.223 16.683 1.207 18.300
family.Forexample,theconcentrationofisoflavonesinsoyseeds,
the most consumed source of isoflavone in the world, varies
between0.5 and 2.0mg/g, while thelowest isoflavone content
foundinT.riograndense wastherootswith2.806mg/g andthe highestwasintheleaveswith7.331mg/g(USDA,2002).
PreviousresearchhasquantifiedisoflavonesinotherTrifolium
species.Ramosetal.(2008)analyzedfivepopulationsofredclover (T.pratense)andobservedthataglyconecontentvariedbetween 0.008and0.091mg/gexpressedindaidzein,0.05and0.131mg/g ingenistein,6.568and23.462mg/ginformononetin,and2.499and 10.337mg/ginbiochaninA.Ingeneral,thedaidzein(3)and genis-tein(4)contentsofT.riograndensearehigherthaninT.pratense, whiletheconcentrationofbiochaninA(1)inT.riograndenseislower (1.207mg/g).
Ascreeningof57Trifoliumspeciesforisoflavoneconcentration showedthatseveralpresentextremelyhighamountsofthese com-pounds.Fromthispointofview,elevenspecies,T.lappaceum,T. phleoides,T.hirtum,T.alpestre,T.medium,T.subterraneum,T. hel-dreichianum,T.pratense,T.isodon,T.miegeanum,andT.scabrum, are interesting,withisoflavone contents ranging from10.39to 88.38mg/g.Mostoftheother46Trifoliumspecieswereeitherfree ofisoflavonesorpresentedverylowcontentsofthesecompounds (Oleszeketal.,2007).
Table2
Invitrochemotacticresponseofneutrophilstreatedwiththesuspensionofdry extractofTrifoliumriograndenseisoflavones.
Sample Concentration
(g/ml)
Distancemigrated (m)
%Inhibition
Control 115±2 100
Trifoliumriograndense 100 10±1* 91
50 9±1a 92
25 8±1a 93
10 8±1a 93
5 7±1a 94
1 13±1a 89
0.5 76±4a 34
0.2 120±1a 0
Daidzein 10 25±1a 78
Genistein 10 55±3a 52
Formononetin 10 51±4a 55
BiochaninA 10 9±1a 92
Theresultsofdistancemigratedaremean±SEM.
ap<0.005,significantlydifferentfromcontrolbyStudent’st-test.
Regardingtheanti-inflammatoryactivityofdryextractofleaves
T.riograndense,wehaveinhibitoryactivityoftheextractagainst neutrophil migration is shown in Table2. All extracts concen-trations(0.2–50.0g/ml)showedadose-dependentinhibitionof neutrophilmigration.Except forthe0.2g/mlextract,allother extractsinhibitedthephenomenonbymorethan90%(p<0.005). Inaddition,isolated isoflavoneswerealsoassayed,allof which significantlyinhibitedneutrophilmigration,especiallybiochanin A(1),whichwasabletoinhibitchemotaxisby92%followedby 78%ofdaidzein(3).However,themajorisoflavone,formononetin (2),didnotpresentthestrongestanti-inflammatoryactivity,and theanti-inflammatorycapacityoftheextractofT.riograndenseis mainlyrelatedtotheclassoftheisoflavonecompoundspresentas observedinthisstudy.
Indeed, all of these isoflavones have been object of anti-inflammatoryinvestigationandtheresultsareinagreementwith theliterature. Previous study hasdemonstrated that biochanin A antagonizes the IL-1-induced catabolic effects through its anti-inflammatoryactivitybythemodulationofNFBsignaling, resultinginpotentanti-inflammatory,anti-catabolic,and antiox-idativeeffectsthroughantagonisticeffectsagainstIL-1binprimary ratchondrocytes.SuchresultssuggestthatbiochaninAmaybean importantphytoestrogentopreventosteoarthritis(Ohetal.,2016). Inotherstudy,biochaninAshowedprotectiveeffecton LPS/GalN-inducedliverinjury,bytheprotectionagainstLPS/GalN-induced liverinjurybyactivatingtheNrf2pathwayandinhibitingNLRP3 activation(Liu etal.,2016b).Formononetin(1)demonstrated a reductionin someinflammatory mediatorssuchasnuclear fac-torB(NF-B)andIL-1invitro(Wangetal.,2012).Inaddition, thiscompoundwasabletodecreasethelevelsofTNF-␣and IL-6(Liet al.,2014)and improvesuperoxidasedismutaseactivity (Maetal.,2013),demonstratingananti-inflammatoryand antiox-idantactivitiesassociatedwithneuronandlungprotectiveeffects
invivo.Inordertostudytheeffectofdaidzeinforthetreatment ofboneloss,thiscompoundwastestedontheexpressionofthe osteoblast-producedboneregulatoryfactorsOPG,RANKLand IL-6 in human osteoblastic MG-63 cells. The resultsshowed that daidzeinincreasedthelevelsofOPGanddecreasedthoseofRANKL andIL-6(Sunetal.,2016).Genisteinwasalsosubmittedfor inves-tigationofitsanti-inflammatoryactivityandtheresultsconfirmed animportantdecreaseintheTNF-␣andIL-6 levels(Inciretal., 2016).
Thesuppressionofneutrophilfunctionsisoneofthewaysto controlinflammatoryconditions.Therelationshipbetween tradi-tionaluseofaplantandaninflammatoryprocesshasbeenstudied usingseveralspecies.TheBoydenchamberisasimpleandefficient
Table3
EffectoforaladministrationofTrifoliumriograndenseextractonratpawedema inducedbycarrageenan(n=5animals).
Treatment Volumeofpawedema(ml)±SEM(%inhibition)
1h 2h 3h 4h
Control 1.19±0.15 1.44±0.23 2.13±0.17 1.69±0.26
Indomethacin 0.12±0.23a 0.47±0.27a 0.72±0.35a 0.48±0.48
10mg/kg (89.9%) (67.4%) (66.2%) (71.6%)
T.riograndense 0.38±0.24a 0.66±0.26 1.13±0.19a 0.58±0.26a
100mg/kg (68.1%) (54.2%) (46.9%) (65.7%)
ap<0.05,significantlydifferentfromcontrolbyStudent’st-test.
methodtodeterminewhetheranisolatedcompoundorextracthas theabilitytoinhibitneutrophilchemotaxis(Suyenagaetal.,2011). Theresultsobtainedintheanalysisofneutrophilchemotactic migrationpromptedustoevaluatetheinvivoantiedematogenic activityoftheextractofT.riograndense.Intheratpawedema,the inflammatoryeffectinducedbyinjectionofcarrageenan0.5%, pro-ducededemaafter60min.Thepreviousadministration(60min)of theextractofT.riograndense100mg/kginducedsignificant inhibi-tion,whencomparedwiththecontrol(p<0.05)(Table3).Asshown inTable3,theextractinhibitedpawedemaalreadywithinthefirst houroftheexperiment(54.2–68.1%ofinhibition).
Considering the results obtained in the anti-inflammatory assays,thefindingsindicatedthattheextractobtainedfromthe leavesshowedanti-inflammatoryandantiedematogenic propri-etiesduetoreductionofacuteedema.
Isoflavones have attractedattentiondue to theirrole in the amelioration of postmenopausal symptoms, cardiovascular dis-eases,cognitivefunction,andbreastandprostatecancers(Verheus etal.,2007).Isoflavone-basednutraceuticalsarethemostassayed polyphenol supplements. The interest in isoflavones as dietary componentsandtheirscarcityinWesterndietsascompared to Asian diets,where theyare abundantdue tosoy consumption, hasresultedinincreasingdemandfornewplantsourcesofthese compounds(Wangetal.,2013).
The model of chemotaxis is simulated byin vitro leukocyte migrationfromtheintravascularspaceintothetissue(Hofbauer etal.,1998).Thechemotaxisassayevaluatesthedecreasein motil-ityofleukocytechemotacticagentsahead,andconsidersthespace traversedbyleukocytemigrationasameasureofactivity.Itshould be emphasized that the reading of cell migration is observed
invitroandthereforeisnotameasureofthedistancemigrated
invivo,thoughbothparametersarehighlycorrelated.Based on theknowledgethatneutrophils,inparticularleukocytes,playan importantroleintheinflammatoryprocess,itmaybesuggested thatinhibitionoftheirmigrationmayberesponsibleforpartofthe anti-inflammatoryactivity.Thesuppressionofneutrophilfunction cancontroltheinflammatoryresponsebeingappliedasa mech-anismofaction ofcertainanti-inflammatorydrugs (Riojaetal., 2000).
This test demonstrated the action of dry extract from T. riograndense leavesonleukocytesmigration, provingthe occur-renceofchemotaxisinhibition.Thiseffectonthemigrationcaused by the extract of this species of clover can be attributed to isoflavones. The fact that the extract of T. riograndense caused greaterinhibitiononleukocytechemotaxis,whencomparedwith theisolatedisoflavones,suggeststheoccurrenceofsynergism,in whichtheactionof twoormoreisoflavonesmaycauseamore intenseeffect.
Thepawedema inducedby carrageenan followsa model of acuteinflammationthatconsistsoftwophases:thefirst,which wasdetectedafteraround1handwascalledthefastphase,with releaseofhistamineandserotonin,andthesecondstage,calledlate, withthemediators(kinins,prostaglandins)releasedafter2and3h, respectively(Vinegaretal.,1969;DiRosaetal.,1971).Inamodel ofinducedarthritis,Trifoliumresupinatumvar.microcephalumwas showntohaveactivityinratpawedema(Sabudaketal.,2008). ChemicalanalysesshowedthatT.riograndense isaplantrichin isoflavonesandanewinterestingoptionasisoflavonesource.The resultsofthebiologicalteststakentogethershowthattheextract ofT.riograndensehasanti-inflammatoryproperty.However,the cellularmechanismsinvolvedinthisactivitydeservefurtherstudy. Othertests,invivoandinvitro,arenecessarytoconfirmtheresults, andfurtherinvestigationscouldindeedestablishprobable mecha-nismsofaction.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Author’scontribution
GPRP,CBM,GRDandPCCcarriedoutthephytochemical pro-cess.GPRPandGRDmadethechromatographicassays.MAAand JASZcontributedinwritingthemanuscript.MAA,EESSandGRD performedthebiologicalanalysis.MDAhelpedinthecollection andidentificationoftheplantmaterial.MAAandEESScontributed tothecriticalreadingthemanuscript.Allauthorshaveapproved thefinalversionforpublishing.
Acknowledgements
Thisinvestigationwassupportedbygrantsof FAPERGS, and CNPq.WearegratefultotheCNPqforthefellowshipsupport.
References
Burkart,A.,1987.TrifoliumL.In:FloraIlustradadeEntreRios(Argentina).I.N.T.A., Argentina,p.219–662.
Cavendish,R.L.,Santos,J.S.,Neto,R.B.,Paixão,A.O.,Oliveira,J.O.,deAraujo,E.D.,Silva, A.A.B.,Thomazzi,S.M.,Cardoso,J.C.,Gomes,M.Z.,2015.Antinociceptiveand anti-inflammatoryeffectsofBrazilianredpropolisextractandformononetin inrodents.J.Ethnopharmacol.173,127–133.
DiRosa,M.,Giroud,J.P.,Willoughby,D.A.,1971.Studiesonthemediatorsofthe acuteinflammatoryresponseinducedinratsindifferentsitesbycarrageenan andturpentine.J.Pathol.104,15–29.
Gillet,J.M.,Collins,M.,Taylor,N.J.,2001.TheWorldofClovers.IowaStateUniversity Press,Ames,pp.457pp.
Honmore,V.S.,Kandhare,A.D.,Kadam,P.P.,Khedkar,V.M.,Sarkar,D.,Bodhankar, S.L.,Zanwar,A.A.,Rojatkar,S.R.,Natu,A.D.,2016.IsolatesofAlpiniaofficinarum HanceasCOX-2inhibitors:evidencefromanti-inflammatory,antioxidantand moleculardockingstudies.Internat.Immunopharmacol.33,8–17.
Hofbauer,R.,Moser,D.,Salfinger,H.,Frass,M.,Kapiotis,S.,1998.Sufentanilinhibits migrationofhumanleukocytesthroughhumanendothelialcellmonolayers. Anesth.Analg.87,1181–1185.
Incir,S.,Bolayirli,I.M.,Inan,O.,Aydın,M.S.,Bilgin,I.A.,Sayan,I.,Esrefoglu,M.,Seven, A.,2016.Theeffectsofgenisteinsupplementationonfructoseinducedinsulin resistance,oxidativestressandinflammation.LifeSci.158,57–62.
Ji,G.,Zhang,Y.,Yang,Q.,Cheng,S.,Hao,J.,Zhao,X.,Jiang,Z.,2016.Genistein sup-pressesLPS-inducedinflammatoryresponsethroughinhibitingNF-kBfollowing AMPkinaseactivationinRAW264.7macrophages.PLoSOne7(12),e53101.
Lemeziene,N.,Padarauskas,A.,Butkute,B.,Ceseviciene,J.,Taujenis,L., Norke-viciene,E., Mikaliuniene,J.,2015. Theconcentrationofisoflavones inred clover(TrifoliumpratenseL.)atfloweringstage.Zemdirbyste-Agriculture102, 443–448.
Li,Z.,Dong,X.,Zhang,J.,Zeng,G.,Zhao,H.,Liu,Y.,Qiu,R.,Mo,L.,Ye,Y.,2014. For-mononetinprotectsTBIratsagainstneurologicallesionsandtheunderlying mechanism.J.Neurol.Sci.15,112–117.
Liu,D.,Cao,G.,Han,L.,Ye,Y.,SiMa,Y.,Ge,W.,2016a.FlavonoidsfromRadix TetrastigmaeinhibitTLR4/MD-2mediatedJNKandNF-Bpathwaywith anti-inflammatoryproperties.Cytokine84,29–36.
Liu,X.,Wang,T.,Liu,X.,Cai,L.,Qi,J.,Zhang,P.,Li,Y.,2016b.BiochaninAprotects lipopolysaccharide/d-galactosamine-inducedacuteliverinjuryinmiceby acti-vatingtheNrf2pathwayandinhibitingNLRP3inflammasomeactivation.Int. Immunopharmacol.38,324–331.
Ma,Z.,Ji,W.,Fu,Q.,Ma,S.,2013.FormononetininhibitedtheinflammationofLPS inducedacutelunginjuryinmiceassociatedwithinductionofPPARgamma expression.Inflammation36,1560–1566.
Oh,J.S.,Cho,I.A.,Kang,K.R.,You,J.S.,Yu,S.J.,Lee,G.J.,Seo,Y.S.,Kim,C.S.,Kim, doK.,Kim,S.G.,Seo,Y.W.,Im,H.J.,Kim,J.S.,2016.Biochanin-Aantagonizes theinterleukin-1-inducedcatabolicinflammationthroughthemodulationof NFkBcellularsignalinginprimaryratchondrocytes.Biochem.Biophys.Res. Commun.477,723–730.
Oleszek,W.,Stochmal,A.,Janda,B.,2007.Concentrationofisoflavonesandother phenolicsintheaerialpartsofTrifolium species.J. Agric.FoodChem.55, 8095–8100.
Ramos,G.P.,Dias,P.M.B.,Morais,C.B.,Froehlich,P.E.,Dall’Agnol,M.,Zuanazzi,J.A.S., 2008.LCdeterminationoffourisoflavoneaglyconesinredclover(Trifolium pratenseL.).J.Chromatogr.67,125–129.
Rioja,I.,Ubeda,A.,Terencio,M.C.,Guillen,I.,Riguera,R.,Quintela,J.M.,Peinador,C., Gonzalez,L.M.,Alcaraz,M.J.,2000.Ananti-inflammatoryditriazineinhibiting leukocytefunctionsandexpressionofinduciblenitricoxidesynthaseand cyclo-oxygenase-2.Eur.J.Pharmacol.397,207–217.
Sabudak,T.,Dokmeci,D.,Ozyigit,F.,Isik,E.,Aydogdu,N.,2008.Anti-inflammatory andantioxidantactivitiesofTrifoliumresupinatumvar.microcephalumextracts inarthriticrats.AsianJ.Chem.20,1491–1496.
Sahpaz,F.,Dogukan,A.,Dagli,M.N.,2016.Effectofisoflavonesonatherosclerosisin peritonealdialysispatients.CukurovaMed.J.41,112–120.
Sun,J.,Sun,W.J.,Li,Z.Y.,Li,L.,Wang,Y.,Zhao,Y.,Wang,C.,Yu,L.R.,Li,L.Z.,Zhang, Y.L.,2016.DaidzeinincreasesOPG/RANKLratioandsuppressesIL-6inMG-63 osteoblastcells.Int.Immunopharmacol.40,32–40.
Suyenaga,E.S.,Konrath,E.L.,Dresch,R.R.,Apel,M.A.,Zuanazzi,J.A.,Chaves,C.G., Henriques,A.T.,2011.Appraisaloftheantichemotacticactivityofflavonoidson polymorphonuclearneutrophils.PlantaMed.77,698–704.
Tava,A.,Pecio,L.,Stochmal,A.,Pecetti,L.,2015.Clovamideandflavonoidsfrom leavesofTrifoliumpratenseandT.pratensesubspnivalegrowninItaly.Nat.Prod. Commun.10,933–936.
USDA–UnitedStatesDepartmentofAgricultureAgriculturalResearchService &IowaStateUniversity,2002.USDA-IowaStateUniversityDatabaseonthe IsoflavoneContentofFoods.
Verheus,M.,VanGils,C.H.,Keinan-Boker,L.,Grace,P.B.,Bingham,S.A.,Peeters, P.H.M.,2007.Plasmaphytotoestrogenandsubsequentbreastcancerrisk.J.Clin. Oncol.25,648–655.
Vinegar,R.,Schreiber,W.,Hugo,R.,1969.Biphasicdevelopmentofcarrageenin edemainrats.J.Pharmacol.Exp.Ther.166,96–103.
Wang,Y.,Zhu,Y.,Gao,L.,Yin,H.,Xie,Z.,Wang,D.,Zhu,Z.,Han,X.,2012. For-mononetinattenuatesIL-1-inducedapoptosisandNF-BactivationinINS-1 cells.Molecules17,10052–10064.
Wang,Q.,Ge,X.,Tian,X.,Zhang,Y.,Zhang,J.,Zhang,P.,2013.Soyisoflavone:the multipurposephytochemical(Review).Biosci.Rep.1,697–701.
Winekenstadde,D.,Angelis,A.,Waltenberger,B.,Schwaiger,S.,Tchoumtchoua,J., Konig,S.,Werz,O.,Aligiannis,N.,Skaltsounis,A.L.,Stuppner,H.,2015. Phyto-chemicalprofileoftheaerialpartsofSedumsediformeandanti-inflammatory activityofmyricitrin.Nat.Prod.Commun.10,83–88.
Winter,C.A.,Risley,E.A.,Nuss,G.W.,1962.Carragenin-inducedoedemainhindpaw oftheratasanassayforanti-inflammatorydrugs.P.Soc.Exp.Biol.Med.111, 244–247.
Wu,Q.,Wang,M.,Simon,J.E.,2003.Determinationofisoflavonesinredclover andrelatedspeciesbyhigh-performanceliquidchromatography combined withultravioletandmass spectrometricdetection.J. Chromatogr.A1016, 195–209.