w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Polysaccharide
rich
fractions
from
barks
of
Ximenia
americana
inhibit
peripheral
inflammatory
nociception
in
mice
Antinociceptive
effect
of
Ximenia
americana
polysaccharide
rich
fractions
Kaira
E.S.
da
Silva-Leite
a,
Ana
M.S.
Assreuy
a,∗,
Laryssa
F.
Mendonc¸
a
a,
Luis
E.A.
Damasceno
a,
Maria
G.R.
de
Queiroz
b,
Paulo
A.S.
Mourão
c,
Alana
F.
Pires
a,
Maria
G.
Pereira
a,daInstitutoSuperiordeCiênciasBiomédicas,UniversidadeEstadualdoCeará,Fortaleza,CE,Brazil
bDepartamentodeAnálisesClínicaseToxicológicas,UniversidadeFederaldoCeará,Fortaleza,CE,Brazil
cInstitutodeBioquímicaMédica,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
dFaculdadedeEducac¸ão,CiênciaseLetrasdoSertãoCentral,UniversidadeEstadualdoCeará,Quixadá,CE,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received7June2016 Accepted7December2016 Availableonline24January2017
Keywords:
Antinociceptiveactivity Structuralcharacterization Medicinalplant Plantpolysaccharides Purification Toxicity
a
b
s
t
r
a
c
t
XimeniaamericanaL.,Olacaceae,barksareutilizedinfolkmedicineasanalgesicandanti-inflammatory. Theobjectivewastoevaluatethetoxicityandantinociceptiveeffectofpolysaccharidesrichfractionsfrom
X.americanabarks.ThefractionswereobtainedbyextractionwithNaOH,followedbyprecipitationwith ethanolandfractionationbyionexchangechromatography.Theywereadministeredi.v.orp.o.before nociceptiontests(writhing,formalin,carragenan-inducedhypernociception,hotplate),orduring14days fortoxicityassay.Thetotalpolysaccharidesfraction(TPL-Xa:8.1%yield)presented43%carbohydrate (21%uronicacid)andresultedintwomainfractionsafterchromatography(FI:12%,FII:22%yield).FII showedbetterhomogeneity/purity,contentof44%carbohydrate,including39%uronicacid,arabinose andgalactoseasmajormonosaccharides,andinfraredspectrawithpeaksincarbohydraterangeforCOO− groupsofuronicacid.TPL-Xa(10mg/kg)andFII(0.1and1mg/kg)presentedinhibitoryeffectinbehavior teststhatevaluatenociceptioninducedbychemicalandmechanical,butnotthermalstimuli.TPL-Xadid notalterparametersofsystemictoxicity.Inconclusion,polysaccharidesrichfractionsofX.americana
barksinhibitperipheralinflammatorynociception,beingwelltoleratedbyanimals.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Theuseofmedicinalplantsasanalgesicagentsisa common
practicethathaspromptedethnopharmacologicalstudies.Among
plantconstituents,polysaccharidesarefoundinlargequantities
andshowlowtoxicity(Ovodov,1998).
XimeniaamericanaL.,Olacaceae,isdistributedintropicaland
temperateregions,beingpopularlyknownintheNortheastBrazil
as“ameixa-do-mato”,“ameixa-brava”or“ameixa-do-sertão”(Silva
etal.,2008).InBrazilian folkmedicineasinothercountries, X. americanabarksareutilizedasanti-cancer,analgesicforheadaches,
∗ Correspondingauthor.
E-mail:ana.assereuy@uece.br(A.M.Assreuy).
gastric and back pains and other inflammatory conditions (de
Albuquerque et al., 2007; Le et al., 2012). The phytochemical
analysisofX.americanabarks extractsrevealedthepresenceof
alkaloids,anthraquinones,glycosides,flavonoids,saponins,tannins terpenoids(Maikaietal.,2010)and carbohydrates(Jamesetal.,
2007).Experimentalstudiesperformedwiththeaqueousextracts
ofthisplanthaddemonstratedantinociceptiveactivity(Soroetal., 2009).
Theimmunomodulatoryroleofplantpolysaccharidesisalready
welldescribed(SchepetkinandQuinn,2006),includingthe
anti-inflammatoryeffect(Pereiraetal.,2012a,b).However,theeffectof plantpolysaccharidesinthenociceptionprocessisscarce,although
recent studieshad demonstratedthe antinociceptiveactivityin
mice for Thladiantha dubia crude polysaccharides (Wanget al.,
2011),andforarabinogalactan(doNascimentoetal.,2015)and
http://dx.doi.org/10.1016/j.bjp.2016.12.001
galactoarabinoglucuronoxylan from Solanumbetaceum fruit (do Nascimentoetal.,2013).Inthisstudypolysacchariderichfractions ofX.americanabarkswereevaluatedinmiceforitstoxicityand antinociceptiveeffect.
Materialsandmethods
Animals
MaleSwissmice(20–25g),5–6weeksofage,weremaintained
withfreeaccesstowaterandfoodat22–26◦C,12/12hlight/dark
cycle.TheexperimentalprotocolswereapprovedbytheAnimal
Care and Use Committee of the State University of Ceará (n◦
12783679-9/2012).
Drugsandreagents
DEAE-cellulose, indomethacin, bovine serum albumin (BSA),
-carrageenan (Cg) and monosaccharides (Sigma Chemical Co.,
St.Louis,MO,USA);agarose(BioRadLaboratories);N-cetyl-N-N
-N-trimethylammonium bromide(Cetavlon) (British Drug House
Chemical, Ltd.); chondroitin-6-sulfate, heparin sulfate and
der-matansulfate(SeikagauKogyoCo);morphine(Dimorf®,Cristalia,
SP,Brazil);diazepam(TeutoS/A,GO,Brazil);formaldehyde and
aceticacid(Isofar,RiodeJaneiro,RJ,Brazil);ketamineandxylazine
(KönigS/A,Argentina).Theremainingdrugsandreagentswereof
analyticalgrade.
Plantcollection,polysaccharidesextractionandfractioning
Ximenia americana L., Olacaceae, was collected at
Custódio-Quixadá,Ceará(Brazil)and avoucherspecimen(n◦ 46794)was
deposited in the Herbarium Prisco Bezerra of Federal
Univer-sityofCeará.BarksofX. americanawerewashed,driedat40◦C
andmaceratedintopowder(5g).Thepowderwassuspendedin
methanol(1:50,w/v,76◦C,2h),toremovepigments,andfiltered
(steprepeatedtwice).Theinsolubleresiduewasaddedto0.1M
NaOH(1:50,w/v,97◦C,2h),filtered(steprepeatedthreetimes)
andcentrifuged (2496×g;15min,25◦C).Alkalinesupernatants
werepooled,neutralizedin1MHClandprecipitatedinethanol
(1:4(w/v);24h,4◦C).Themixturewascentrifugedandthe
pel-letwasdialyzed(cut-off14,000Da;72h)againstdistilled water
andre-centrifuged(Pereiraetal.,2016).Thefinalsupernatantwas
lyophilizedandnamedtotalpolysaccharidesofX.americana
(TPL-Xa).
TPL-Xa(1:2,w/v)wasdissolvedindistilledwaterandapplied
to ion exchange chromatography – DEAE-cellulose. Column
(9.8×2.0cm)wasequilibratedandelutedwithdistilledwaterfor
removal of neutral polysaccharides, and the acidic
polysaccha-rideswereeluted(1ml/min)withNaCl(0.1,0.25,0.5,0.75,1.0M).
Polysaccharidefractionsweremonitoredforthecarbohydrate
con-tentbythemethodofphenol–sulfuricacid(DuBoisetal.,1956).
Polysaccharidescharacterization
Polysaccharideswerequantifiedfortotalcarbohydrate(DuBois
et al., 1956), uronic acid (Dische, 1947) and soluble
pro-tein (Bradford, 1976), using arabinose and galactose (3:1),
d-galacturonicacidandBSA(albuminserumbovine)asrespective
standards.
Agarose 0.5% gel electrophoresis: polysaccharides (6mg/ml,
15l) were applied and run in 0.05M
1,3-diaminopropane-acetatebuffer(pH 9.0)for60minat110Vand fixedwith0.1%
Cetavlonfor24h.GelwasdriedandstainedwithStains-All(5mg
Stains-All; 100ml of 50% ethanol, w/v) and washed with
dis-tilledwater(DietrichandDietrich,1976;Souzaetal.,2015).The
glycosaminoglycans chondroitin 6-sulfate (∼60kDa), dermatan
sulfate(∼30kDa)andheparansulfate(∼15kDa)wereusedas stan-dards.
Themonosaccharidecompositionwasanalyzedbygas–liquid
chromatographycoupledtomassspectrometry(GC–MS).
Polysac-charidefractions(5mg)werehydrolyzedwithtrifluoroaceticacid
(1mol/l; 96◦C; 5h) evaporated in rota evaporator (Buchi RE
11, Switzerland), extensively washed with water and reduced
with sodium borohydride (1h, r.t.). The reaction was
inter-ruptedwithacetic aciduntilneutralization.The resultingboric
acidwasremovedas trimethylboratewithmethanol(3×5ml)
in the rota evaporator and acetylation carried out with acetic
anhydride–pyridine(1:1(v/v); 100◦C;1h).Theresultingalditol
acetate was extracted with chloroform (5ml) and analyzed by
GC–MSHP-Ultra2column(Kircher,1960).
Polysaccharideswereanalyzedbyinfrared(FTIR)spectroscopy
(Bruker–Vertex70),coupledtoPikeMiraclesingle-bounce attenu-atedtotalreflectance(ATR)cellequippedwithaZnSesinglecrystal
module.Thespectralregionexaminedextendedof500–4000cm−1
usingaresolutionof3cm−1.Allspectraaretheaverage
measure-mentswith124scanseach.
Toxicityassay
Mice were weighed beforeand after treatmentfor 14 days
withTPL-Xa(10mg/kg, i.v.). Bloodwascollectedafter
anesthe-sia intraperitoneal (i.p.) with ketamine 90mg/kg and xylazine
10mg/kgforhematologicalanalysis(erythrocytes,leukocytesand
platelets),serum contentof ureaand creatinineand enzymatic
activityofalaninetransaminase(ALT)andaspartatetransaminase
(AST).Heart,spleen,stomach,kidneyandliverwereremovedand
weighed(wetweight/bodymass).
Behavioraltests
Mice(n=6−8pergroup)weretreated30minbeforetestswith polysaccharidesi.v.(0.1,1,10mg/kg)orp.o. (100mg/kg),sterile saline(0.9%NaCl;0.05ml/10gbodymass;i.v.),morphine(5mg/kg,
s.c.),indomethacin(10mg/kg,i.p.)ordiazepam(5mg/kg,i.p.).The
protocolswereconductedinadouble-blindmanner.
Formalintest:formalin(2.5%v/v;20l)wasinjecteds.c.inthe
hindanimalpawsandthetime(s)inwhichtheyspentlickingits
pawsinresponsetochemicalstimuliwasrecordedintheinitial
(P1:0–5min)andlate(P2:15–30min)phases(LeBarsetal.,2001). Writhingtest:aceticacid(0.8%(v/v);0.1ml/10gbodymass)was injectedi.p.andthenumberofwrithes(typicalcontractionsofthe
abdominalmusculaturefollowedbyhindlimbstretches),elicited
inresponsetochemicalstimuli,wascountedfrom10to30min
post-injection(LeBarsetal.,2001).
Carrageenan-inducedpawhypernociception:carrageenanwas
injected by intraplantar route(500g/paw, s.c.). Animalswere
placedinclearacrylicboxeswithraisedplatformsofwiremeshto
allowaccesstotheventralsurfaceofhindpawsfrom15to30min
beforeevaluation.Forthis,thefrequencyofpawwithdrawalwas
quantifiedat10sintervalsaftersixapplicationsofstimuli(100%), using0.8gflexiblevonFreyfilaments,attimezeroandfrom1-3h afterstimulationwithcarrageenan(LeBarsetal.,2001).
Hotplatetest:animalswereplacedonahotplateat55±0.5◦C
andthetimedelayedbeforebehavioralresponses(shaking,
lick-ing paws or jumping) was recorded at baseline and after 30,
60–150min.Animalsreactiontimehigherthan10s24hpriortest
wasexcluded.
Rota-rodtest:animalswereselected24hpriortest,excluding
0.389
A
B
C
0.3390.289
0.239
0.189
0.139
+
0.089
0.0
0.1
0.2
0.3
0.4
0.5
0.6 1
4000 3000
3361 2916
2850
1576 1396
1030 1735
2000 1000
3500 2500 1500 500
12
0.1M 0.25M
Fractions (ml)
Wavenumber (cm–1)
FI
FII
23 34 45 56 67 78 89
Origin CS-60 kDa
DS-30 kDa
HS-15 kDa
TPL-Xa
FI FII – A490 nm
T
ransmittance %
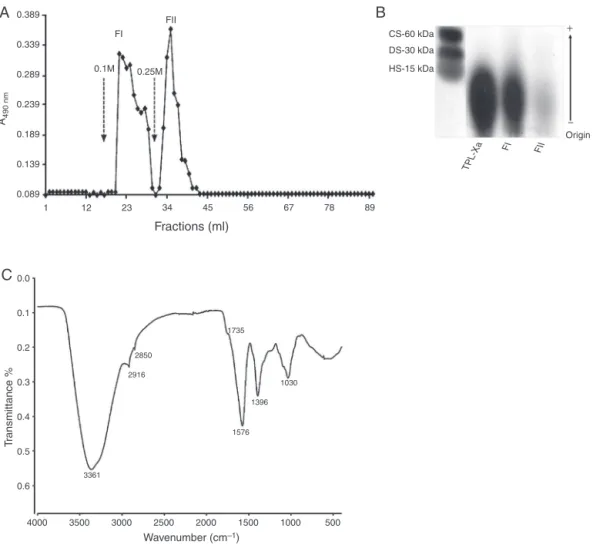
Fig.1.PartialpurificationandcharacterizationofpolysaccharidesfromXimeniaamericanabarks.(A)TPL-Xa(10mg)wasappliedtoDEAE-cellulosecolumn(9.8cm×2.0cm) andresinelutedwithwater.Theacidicpolysaccharidefractionswereeluted(1ml/min)bystepwiseinNaCl(↓)andmonitoredfortotalcarbohydratesatA490nm()by
thephenol-sulfuricacidicmethod.(B)TPL-Xa,FIorFII(6mg/ml,15l)wereappliedin0.5%agarosegel0.05M1,3-diaminopropane:acetatepH9.0(110V,60min)and stainedwithStains-All.Chondroitin6-sulfate(CS);Dermatansulfate(DS)andHeparansulfate(HS).(C)FIIwasanalyzedbyinfrared(FTIR)spectroscopy.Thespectralregion examinedwasfrom500to4000cm−1withresolutionof3cm−1.
consecutiveperiodsof60s.Thepermanencytimeinapparatuswas
quantified(D’amourandSmith,1941).
Statisticalanalysis
Resultsarepresentedasmean±S.E.Mandanalyzedby
One-wayANOVAandBonferronitest(Prism5.0,GraphPadSoftwareInc.,
California,USA).Valuesofp<0.05wereconsideredsignificant.
Resultsanddiscussion
TheextractionoftotalpolysaccharidesfromX.americanabarks
(TPL-Xa)revealed8.1%yieldandpresentedhighcarbohydrate
con-tent(43%,including21%uronicacid)withlowprotein(6.5%).The
extractionof TPL-Xashowed higher yield and similar
carbohy-dratecontentcomparedtothoseobtainedfromotherterrestrial
Angiosperm, whose primary cell walls are composedby pectic
polysaccharides,suchasAzadirachtaindica(1.3%,54%),Caesalpinia ferrea(2.8%,31%)andErigeroncanadensis(1%,34.1%),extractedby similarprocedures(Pereiraetal.,2012a,b;Pawlaczyketal.,2011).
FractioningofTPL-Xa(DEAE-cellulose)resultedintwomajor
peakselutedat0.1(FI:12%yield)and0.25MNaCl(FII:22%yield).
FIIpresentedbetteryieldandhighestresolutioncomparedtoFI
(Fig.1A).Chemicalanalysisofthepolysaccharidefractionsrevealed
highcontentofcarbohydrateinFII(44%totalcarbohydrate,
con-taining39%uronicacid)comparedtoFI(20%totalcarbohydrate,
containing8%uronicacid)(Table1).Inaddition,thecontentof pro-teinswasstillinferior,especiallyinFII(1.6%)comparedtothatof FI(2.4%)andTPL-Xa.
ThecarbohydratecontentofFIIwassimilartoTPL-Xaand
supe-riortoFIandtoFIIofC.ferrea(Pereiraetal.,2012a)andFIIofA.indica
(Pereiraetal.,2012b).Inbothfractionstheproteincontaminant
waslowercomparedtothatofTPL-Xa.
The agarosegel electrophoresisrevealed polydispersebands
typicalofpolysaccharides(Fig.1B)afterstainingwithStains-All, suggestingbetterpurityforFIIandindicativeofuronicacid
pres-ence. Similarfeaturewasdemonstrated forthepolysaccharides
obtainedfromGeoffroeaspinosabarks(Souzaetal.,2015).In
addi-tion,themonosaccharidecomposition byGC–MSdemonstrated
thatpolysaccharidefractionsarecomposedmainlybyarabinose
(FI:39%;FII:57%)andgalactose(FI:16%;FII:20%),however,FIalso
presented35%glucose(Table1).Themonosaccharidecomposition
ofFII,showingbetterhomogeneitythanFI,corroboratesits
rela-tivepurityandwassimilartootherpecticpolysaccharidesisolated fromIlexlatifolia(Fanetal.,2014),E.canadensis(Pawlaczyketal., 2011)andG.spinosa(Souzaetal.,2015).
FTIR-ATRspectraofFII(Fig.1C),themajorpolysaccharide
frac-tion(containing highcontentof carbohydrateand uronicacid),
revealedabsorptionpeaksintheregionof1200–1000cm−1,
cor-respondingtocarbohydraterange(Souzaetal.,2015);signalsat 3361cm−1,assignedto–OHstretchingvibration(Lietal.,2014)and
Table1
CarbohydratecontentandmonosaccharidecompositionofpolysaccharidefractionsofXimeniaamericanabarks,FIandFII.
Fractions Carbohydrate(%) Uronicacid(%) Ara Rha Gal Glc Xyl Man
FI 20 8 39 4 16 35 4 2
FII 44 39 57 7 20 7 9 –
Arabinose(Ara),Rhamnose(Rha),Galactose(Gal),Glucose(Glc),Xylose(Xyl),Mannose(Man)inmolarpercentage.
vibrationofC–Hlinkage,especiallymethyl(CH3)group.Also,itwas
detectedpeaksat1750–1396cm−1,resonancesofCOO−1groupsof
uronicacid(Zhaoetal.,2007;Pawlaczyketal.,2011;Souzaetal., 2015)andat1735cm−1originatedfromtheC Ostretching
vibra-tion,confirmingthepresenceofuronicacid(groupCOOH)inthe
fractionFII(Lietal.,2014).Besides,thelackofsignalsat1240cm−1
indicatedtheabsenceofsulfateestersinFII(Souzaetal.,2015).The FTIR-ATRofFIIcorroboratesthehighcontentofuronicacid
demon-stratedeitherin theagarosegelelectrophoresisorby chemical
analysis.
Somestudieshavebeingestablishingacorrelationbetweenthe
presenceofuronicacid,animportantfeatureofpectic polysaccha-ridesofplantcellwalls(DrozdovaandBubenchikov,2005;Pereira etal.,2012b),andbiologicalactivities,suchasantitussive, antioxi-dant,anti-inflammatoryandanticoagulant(Nosál’ováetal.,2000;
Yoonetal.,2002;Chenetal.,2004).Thiscorrelationwouldalsobe
associatedwiththeantinociceptiveactivitydemonstratedinour
study.
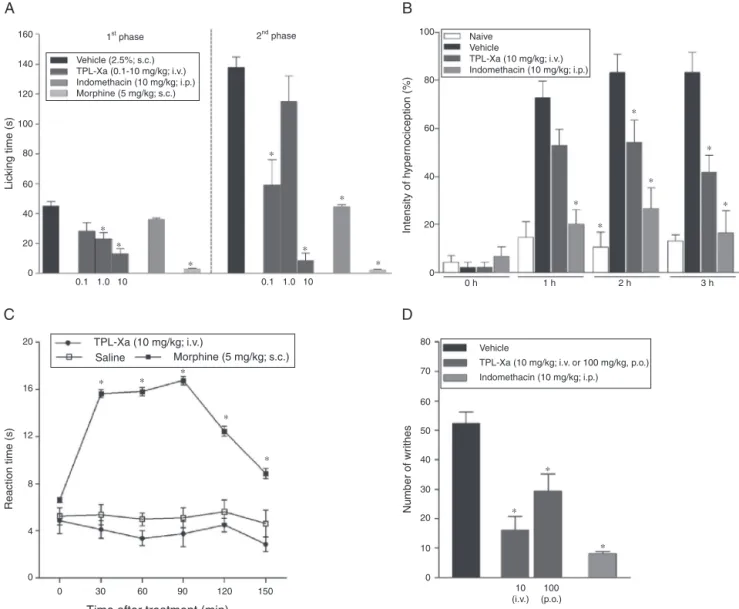
Thei.v.treatmentofanimalswithTPL-Xaproduced
antinocicep-tioninthebehavioralteststhatevaluatechemical(Formalinand
Writhing) and mechanical (carrageenan-induced
hypernocicep-tion),butnotthermal(HotPlate)stimuli.TPL-Xashowedinhibitory effectinthefirstphase(neurogenic)offormalintest,characterized bydirectexcitationofnociceptiveafferentfibers(LeBarsetal., 2001),inhibitingthelickingtimeby48%(23.0±4.4s)at1mg/kg
and by78%(12.8±3.5s)at 10mg/kg. TPL-Xaalsoinhibitedthe
formalinsecondphase(inflammatory),characterizedbyreleaseof
inflammatorymediators(LeBarsetal.,2001),by50%(58.9±16.9s)
at 0.1mg/kg and by 93% (8.1±5.2s) at 10mg/kg compared to
saline (128.7±11.4s) (Fig. 2A). FII decreased the licking time
2nd phase
1st phase
0 20 40 60 80 100
0 20 40 60 80 100
Vehicle (2.5%; s.c.) TPL-Xa (0.1-10 mg/kg; i.v.) Indomethacin (10 mg/kg; i.p.) Morphine (5 mg/kg; s.c.)
TPL-Xa (10 mg/kg; i.v.)
Saline Morphine (5 mg/kg; s.c.)
120 140 160
0.1 1.0 10 0.1 0 h
0 30 60 90 120 150
1 h 2 h 3 h
1.0 10
Time after treatment (min)
Lic
king time (s)
0 4 8 12 16 20
Reaction time (s)
10 (i.v.)
100 (p.o.) 0
10 20 30 40 50 60 70 80
Number of wr
ithes
Intensity of h
yper
nociception (%)
∗
∗ ∗ ∗
∗
∗
∗ ∗
∗ ∗
∗ ∗
∗ ∗ ∗
∗
∗
∗ ∗
∗ ∗
A
B
C
D
Naive Vehicle
TPL-Xa (10 mg/kg; i.v.) Indomethacin (10 mg/kg; i.p.)
Vehicle
TPL-Xa (10 mg/kg; i.v. or 100 mg/kg, p.o.) Indomethacin (10 mg/kg; i.p.)
Fig.2. TPL-Xaantinociceptiveeffect.Micewerepre-treatedwithsaline(i.v.),morphine(5mg/kg;s.c.),indomethacin(10mg/kg;i.p.),TPL-Xa(0.1–10mg/kg;i.v.)orTPL-Xa (100mg/kg;p.o.).(A)Formalin(2.5%;s.c.);(B)Carrageenan-inducedhypernociception(500g/paw;s.c.);(C)Hotplate(55±0.5◦C);(D)Writhes(0.8%aceticacid;i.p.).
Vehicle
FII (0.1-10 mg/kg; i.v.) Indomethacin (10 mg/kg; i.p.) Morphine (5 mg/kg; s.c.)
Naive Vehicle FII (1 mg/kg; i.v.) Indomethacin (10 mg/kg; i.p.)
Vehicle
FII (0.1-10 mg/kg; i.v.) Indomethacin (10 mg/kg; i.p.)
Saline
FII (1 mg/kg; i.v.)
Morphine (5 mg/kg; s.c.) ∗
∗
∗
∗ ∗
∗
∗
∗
∗
∗
∗ ∗
∗
∗ ∗
∗
∗
∗
∗
Licking time (s)
Reaction time (s)
Number of writhes
Intensity of hypernociception (%)
B
A
D
C
0
0 20 40 60 80 100
0 30 60 90 120 150 180 210 240
10 20 30 40 50 60
0.1 1.0 10 0.1 1 10 0.1 1 10
Time after treatment (min)
0 h
0 4 8 12 16 20
30
0 60 90 120 150
1 h 2 h 3 h
2nd phase 1st
phase
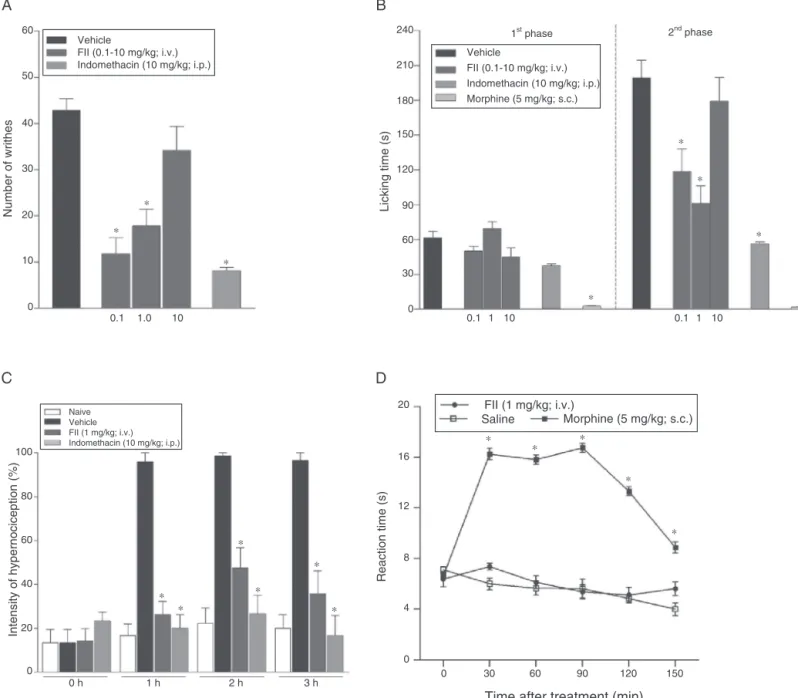
Fig.3.FIIantinociceptiveeffect.Micewerepre-treatedwithsaline(i.v.),indomethacin(10mg/kg;i.p.),morphine(5mg/kg;s.c.),FII(0.1;1or10mg/kg;i.v.).(A)Writhes (0.8%aceticacid;i.p.);(B)Formalin(2.5%;s.c.);(C)Carrageenan-inducedhypernociception(500g/paw;s.c.);(D)Hotplate(55±0.5◦C).Mean±S.E.M.(n=6–8).One-way
ANOVAandBonferronitest.*p<0.05comparedtonociceptivestimuli.
onlyinthesecondphaseby41%at0.1mg/kg(118.5±19.4s)and
60%at1mg/kg(90.7±15.7s)comparedtosaline(199.4±15.3s)
(Fig.3B).Theanalgesicopioidcontrolmorphinereducedthe
lick-ingtime inthefirstand secondphasesby 93%(2.6±0.4s) and
98%(2.1±0.3s),respectively,differingfromtheanti-inflammatory
controlindomethacin,thatinhibitedonlythesecondphaseby68%
(44.3±1.3s)(Fig.2A).TheinhibitoryeffectofFII(richinuronicacid) onlyinthesecondphasesuggeststhatTPL-Xainhibitsthereleaseof
endogenousinflammatorymediatorspartiallymediatedbyacidic
polysaccharidesoffractionFII,andthatthepurificationprocess
increasestheselectivitytoinhibitinflammatorynociception.
Corroboratingthesedata,TPL-XaandFIIreducedthe
hypernoci-ceptiveresponse inducedbycarrageenan.Inmice,theinjection
ofcarrageenanintoanimalpawsinduceshypernociception
char-acterized by the release of inflammatory cytokines, especially
TNF-␣andKC(keratinocyte-derivedchemokine),whichactivate
thereleaseofIL-1(Cunhaetal.,2005)andprostanoids,involved
in the pain sympathetic component (Nakamura and Ferreira,
1987).
Inthistest,TPL-Xa(10mg/kg)inhibitedthefrequencyofpaw
withdrawal atthe2nd hby35% (TPL-Xa:54.1±11.1% vs.
Vehi-cle:83.2±7.7%)andatthe3rdhby50%(TPL-Xa:41.6±8.5%vs.
Vehicle:83.2±11.7%)(Fig.2B).FII(1mg/kg)wasalsoinhibitoryat the1sthby69%(FII:26.1±6.1%vs.Vehicle:86.6±9.7%),atthe 2ndhby52.8%(FII:47.2±9.2%vs.Vehicle:100±0.0%)andatthe 3rdhby63%(FII:35.6±10.5%vs.Vehicle:96.6±3.3%)(Fig.3C).
Indomethacininhibitedthepawwithdrawal atalltimes(1sth:
19.9±6.3;2ndh:26.6±8.5;3rdh:16.6±9.1)(Fig.2B).
IntheWrithingtestTPL-Xainjectedeitheri.v.orp.o.inhibited
thenumberofaceticacid-inducedabdominalwrithes(52.2±3.8)
by 69% (16.1±4.6) at 10mg/kg (i.v.) and 44% (29.3±5.8) at
100mg/kg (p.o.) (Fig. 2D). FII i.v. (0.1 and 1mg/kg) was also
inhibitory (42.8±2.4) by 72% (11.8±3.4) and 58% (17.8±3.6),
Table2
Markersofhepatic,renalandhematologicalfunctionofanimalstreatedwithTPL-Xa.
aTreatment(50l/10g)
Parameters Saline TPL-Xa(10mg/kg) AST(U/l) 65.91±6.15 68.00±3.89 ALT(U/l) 35.45±2.10 35.17±2.14 Urea(mg/dl) 40.31±1.65 38.92±1.59 Creatinine(mg/dl) 0.33±0.01 0.31±0.00 Hematocrit(%) 49.85±1.98 45.15±1.13 Hemoblobin(g/dl) 13.23±0.95 11.50±0.36 Erytrocyte(106ml–1) 8.47±0.24 7.51±0.24
Platelet(103ml–1) 1136±88.68 1213±97.89
Lymphocyte(%) 78.83±1.92 82.00±1.47 Monocyte(%) 0.50±0.34 0.250±0.25 Eosinophil(%) 0.66±0.21 0.0±0.00 Neutrophil(%) 20.14±1.84 16.40±1.36 MCV(fL) 58.81±0.83 60.00±0.43 MCH(pg) 16.65±0.29 16.10±0.12 MCHC(g/dl) 28.38±0.70 26.65±0.41
aMiceweretreateddailyinsingledoseswithTPL-Xa(10mg/kg)orsaline(0.9%)
during14days;Mean±S.E.M(n=7);One-wayANOVAandBonferronitest;*p<0.05 comparedtosaline.ALT,alaninetransaminase;AST,aspartatetransaminase;MCV, meancorpuscularvolume;MCH,meancorpuscularhemoglobin;MCHC,mean cor-puscularhemoglobinconcentration.
writhes(84.4%)(Fig.2D).Thismodelassessesdifferentnociceptive
mechanisms,includingreleaseofinflammatorymediatorssuchas
histamine,serotonin,bradykininandPGE2 (LeBarsetal.,2001).
Theseresultsareinaccordance withtheanti-inflammatoryand
antinociceptiveeffectsofthepolysaccharidesextractedfrom Thla-diantadubia(Wangetal.,2011)andwiththeanti-inflammatory activityofotherpecticpolysaccharides(Salmanetal.,2008).
ThesuggestionofperipheraleffectsofTPL-XaandFIIwere con-firmedbythelackofeffectintheHotplatetest(Figs.2Cand3D), thatevaluatemedullarspinalnociceptivepathways(LeBarsetal., 2001).
It is important to highlight that TPL-Xa (10mg/kg),
differ-ent from the sedative agent (diazepam: 20.1±6.4 vs. saline:
36.4±4.7s),didnotaltertheanimals-fall-latencyintheRota-rod test(TPL-Xa:42.5±5.9svs.saline:36.4±4.7s).Thisdatasuggests thatTPL-Xadoesnotalteranimalsmotoractivity,asideeffect com-monlyassociatedwiththeuseofanalgesicdrugs.Inaddition,mice
treatmentwithTPL-Xaduring14daysdidnotalterthefollowing
parameters:renal,hepaticandhematologicalmarkers(Table2)or theanimalbodymass(initialweight:29.1±1.2vs.finalweight: 31.2±1.4)comparedtosaline(initialweight:28.8±1.0vs.final weight:32.0±1.4).Thewetweighofkidney(TPL-Xa:6.9±0.3vs. Saline:6.5±0.2),stomach(TPL-Xa:8.7±0.5vs.Saline:9.6±0.4),
liver (TPL-Xa: 44.6±0.8 vs. Saline: 42.2±1.0) and heart
(TPL-Xa:4.4±0.1vs.Saline:4.7±0.2),exceptforthespleen(TPL-Xa: 4.1±0.3vs.Saline:2.5±0.1),wasnotaltered.Thesedata
corrobo-ratethewell-knownlowtoxicityofplantpolysaccharides.
In conclusion, polysaccharides rich fractions of X. americana
barks,containinghighlevelsofuronicacid,arabinose,galactoseand
glucose,inhibitperipheralinflammatorynociception,beingwell
toleratedbyanimals.
Authorcontributions
KESSL, LEAD, LFM and AFP conducted animal experiments;
KESSL,MGPandPASMconductedtheextraction,isolationand
char-acterizationofpolysaccharides;MGRQperformedthehematologic
andbiochemicalanalysis;KESSL,AFP,AMSAandMGPsupplied
crit-icalinputtoexperimentaldesignanddatainterpretation;KESSL
providedstatisticalanalysisandinterpretation;KESSL,AFP,AMSA
andMGPwereresponsibleforwritingthemanuscript.Allauthors
havereadandapprovedthesubmissionofthemanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheproceduresfollowedwereinaccordancewiththe
regula-tionsoftherelevantclinicalresearchethicscommitteeandwith
thoseoftheCodeofEthicsoftheWorldMedicalAssociation
(Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata
appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Acknowledgments
This researchwas supported by the fellowships granted by
CAPES,CNPqandFUNCAP.AuthorsthankMsVaneiciaGomesdos
Santosforbotanicalidentification.
References
Bradford,M.M.,1976.Arapidandsensitivemethodforthequantitationof micro-gramquantitiesofproteinutilizingtheprincipleofprotein-dyebinding.Anal. Biochem.72,248–254.
Chen,H.,Zhang,M.,Xie,B.,2004.Quantificationofuronicacidsinteapolysaccharide conjugatesandtheirantioxidantproperties.J.Agric.FoodChem.52,3333–3336.
Cunha,T.M.,Verri,W.A.,Silva,J.S.,Poole,S.,Cunha,F.Q.,Ferreira,S.H.,2005.Acascade ofcytokinesmediatesmechanicalinflammatoryhypernociceptioninmice.Proc. Natl.Acad.Sci.U.S.A.102,1755–1760.
D’amour,F.E.,Smith,D.L.,1941.Amethodfordetermininglossofpainsensation.J. Pharmacol.Exp.Ther.72,74–79.
deAlbuquerque,U.P.,MunizdeMedeiros,P.,deAlmeida,A.L.S.,Monteiro,J.M., MachadodeFreitasLinsNeto,E.,GomesdeMelo,J.,dosSantos,J.P.,2007. Medic-inalplantsofthecaatinga(semi-arid)vegetationofNEBrazil:aquantitative approach.J.Ethnopharmacol.114,325–354.
Dietrich, C.P., Dietrich, S.M.C., 1976. Electrophoretic behaviour of acidic mucopolysaccharidesindiaminebuffers.Anal.Biochem.70,645–647.
Dische,Z.,1947.Anewspecificcolorreactionofhexuronicacidas.J.Biol.Chem.167, 189–198.
doNascimento,G.E.,Corso,C.R.,dePaulaWerner,M.F.,Baggio,C.H.,Iacomini,M., Cordeiro,L.M.C.,2015.Structureofanarabinogalactanfromtheedibletropical fruittamarillo(Solanumbetaceum)anditsantinociceptiveactivity.Carbohydr. Polym.116,300–306.
doNascimento,G.E.,Hamm,L.A.,Baggio,C.H.,Werner,M.F.deP.,Iacomini,M., Cordeiro,L.M.C.,2013.Structureofagalactoarabinoglucuronoxylanfrom tamar-illo(Solanumbetaceum),atropicalexoticfruit,anditsbiologicalactivity.Food Chem.141,510–516.
Drozdova,I.L.,Bubenchikov,R.A.,2005.Compositionandantiinflammatory activ-ityofpolysaccharidecomplexesextractedfromsweetvioletandlowmallow. Pharm.Chem.J.39,197–200.
DuBois,M.,Gilles,K.A.,Hamilton,J.K.,Rebers,P.A.,Smith,F.,1956.Colorimetric methodfordeterminationofsugarsandrelatedsubstances.Anal.Chem.28, 350–356.
Fan,J.,Wu,Z.,Zhao,T.,Sun,Y.,Ye,H.,Xu,R.,Zeng,X.,2014.Characterization, antioxi-dantandhepatoprotectiveactivitiesofpolysaccharidesfromIlexlatifoliaThunb. Carbohydr.Polym.101,990–997.
James, D.B., Abu,E.A., Wurochekke,A.U.,Orji,G.N., 2007. Phytochemicaland antimicrobialinvestigationoftheaqueousandmethanolicextractsofXimenia americana.J.Med.Sci.7,284–288.
Kircher,H.W.,1960.Gas-liquidpartitionchromatographyofmethylatedsugars. Anal.Chem.32,1103–1106.
LeBars,D.,Gozariu,M.,Cadden,S.W.,2001.Animalmodelsofnociception. Pharma-col.Rev.53,597–652.
Le,N.H.T.,Malterud,K.E.,Diallo,D.,Paulsen,B.S.,Nergård,C.S.,Wangensteen,H., 2012.BioactivepolyphenolsinXimeniaamericanaandthetraditionaluseamong Malianhealers.J.Ethnopharmacol.139,858–862.
Li,X.,Jiang,J.,Shi,S.,Bligh,S.W.A.,Li,Y.,Jiang,Y.,Huang,D.,Ke,Y.,Wang,S., 2014.ARG-IItypepolysaccharidepurifiedfromAconitumcoreanumalleviates lipopolysaccharide-inducedinflammationbyinhibitingtheNF-Bsignal path-way.PLoSOne9,e99697,http://dx.doi.org/10.1371/journal.pone.0099697. Maikai,V.A.,Kobo,P.I.,Maikai,B.V.O.,2010.AntioxidantpropertiesofXimenia
Nakamura,M.,Ferreira,S.H.,1987.Aperipheralsympatheticcomponentin inflam-matoryhyperalgesia.Eur.J.Pharmacol.135,145–153.
Nosál’ová,G.,Kardosová,A.,Franová,S.,2000.Antitussiveactivityofa glucuronoxy-lanfromRudbeckiafulgidacomparedtothepotencyoftwopolysaccharide complexesfromthesameherb.Pharmazie55,65–68.
Ovodov,I.S.,1998.Polysaccharidesofflowerplants:structureandphysiological activity.Bioorg.Khim.24,483–501.
Pawlaczyk,I.,Czerchawski,L.,Kuliczkowski,W.,Karolko,B.,Pilecki,W.,Witkiewicz, W.,Gancarz,R.,2011.Anticoagulantandanti-plateletactivityof polyphenolic-polysaccharide preparation isolated from the medicinal plant Erigeron canadensisL.Thromb.Res.127,328–340.
Pereira,L.,de,P.,daSilva,R.O.,Bringel,P.H.,de,S.F.,daSilva,K.E.S.,Assreuy,A.M.S., Pereira,M.G.,2012a.PolysaccharidefractionsofCaesalpiniaferreapods: poten-tialanti-inflammatoryusage.J.Ethnopharmacol.139,642–648.
Pereira,L.,de,P.,Mota,M.R.L.,Brizeno,L.A.C.,Nogueira,F.C.,Ferreira,E.G.M.,Pereira, M.G.,Assreuy,A.M.S.,2016.Modulatoreffectofapolysaccharide-richextract fromCaesalpiniaferreastembarksinratcutaneouswoundhealing:roleofTNF-␣, IL-1,NO,TGF-.J.Ethnopharmacol.187,213–223.
Pereira,L.,de,P.,Silva,K.E.S.da,Silva,R.O.da,Assreuy,A.M.S.,Pereira,M.G.,2012b.
Anti-inflammatorypolysaccharidesofAzadirachtaindicaseedtegument.Rev. Bras.Farmacogn.22,617–622.
Salman,H.,Bergman,M.,Djaldetti,M.,Orlin,J.,Bessler,H.,2008.Citruspectinaffects cytokineproductionbyhumanperipheralbloodmononuclearcells.Biomed. Pharmacother.62,579–582.
Schepetkin, I.A., Quinn, M.T., 2006. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 6, 317–333.
Silva,G.G.da,Souza,P.A.de,Morais,P.L.D.de,Santos,E.C.dos,Moura,R.D.,Menezes, J.B.,2008.Caracterizac¸ãodofrutodeameixasilvestre(XimeniaamericanaL.). Rev.Bras.Frutic.30,311–314.
Soro,T.Y.,Traore,F.,Sakande,J.,2009.Analgesicactivityoftheaqueousextractfrom
Ximeniaamericana.C.R.Biol.332,371–377.
Souza,R.O.S.,Assreuy,A.M.S.,Madeira,J.C.,Chagas,F.D.S.,Parreiras,L.A.,Santos, G.R.C.,Mourão,P.A.S.,Pereira,M.G.,2015.PurifiedpolysaccharidesofGeoffroea spinosabarkshaveanticoagulantandantithromboticactivitiesdevoidof hem-orrhagicrisks.Carbohydr.Polym.124,208–215.
Wang,L.,Zhao,D.,Di,L.,Xu,T.,Lin,X.,Yang,B.,Zhou,X.,Yang,X.,Liu,Y.,2011.The analgesicandanti-rheumaticeffectsofThladianthadubiafruitcrude polysac-charidefractioninmiceandrats.J.Ethnopharmacol.137,1381–1387.
Yoon,S.-J.,Pereira,M.S.,Pavão,M.S.G.,Hwang,J.-K.,Pyun,Y.-R.,Mourão,P.A.S.,2002.
ThemedicinalplantPoranavolubiliscontainspolysaccharideswith anticoagu-lantactivitymediatedbyheparincofactorII.Thromb.Res.106,51–58.