rev bras hematol hemoter. 2017;39(1):57–59
w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Case
Report
Myelodysplasia
and
acute
myeloid
leukemia
fifteen
years
after
high-dose
cyclophosphamide
in
a
child
with
severe
aplastic
anemia
José
Carlos
Jaime-Pérez
∗,
Liliana
Nataly
Guerra-Leal,
Olga
Graciela
Cantú-Rodríguez,
David
Gómez-Almaguer
UniversidadAutónomadeNuevoLeón,FacultaddeMedicina,HospitalUniversitarioDr.JoséE.González,Monterrey,Mexico
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received26May2016 Accepted16June2016 Availableonline12July2016
Introduction
Aplasticanemia(AA)isararedisordercharacterizedby sup-pression of bone marrow function. It can develop as the resultofcongenitalmarrowdiseaseandchemicalexposure; however,mostcasesareidiopathic.1Treatmentwith immuno-suppressive therapy(IST) forpatientswho do nothave an humanleukocyte antigen(HLA)-compatibledonorrelieson the evidence that a deregulated immune system drives T lymphocytestocytokine-mediated destructionoftheirown hematopoietic stem cells.1 The majority of these patients respondwelltoup-frontadministrationofIST,including anti-thymocyte globulin(ATG) and cyclosporine (CsA), which is successfulinaround80%.2 Unfortunately,ATGandCsAcan leadtoclonal disorders,inparticularmyelodysplastic syn-drome(MDS)andparoxysmalnocturnalhemoglobinuria.3On theotherhand,highdosesofcyclophosphamide(HDCY)have been administered as a sole immunosuppressive agent in severeaplasticanemia (SAA),principallyinadults,withno
∗ Correspondingauthorat:HospitalUniversitario“Dr.JoséE.González”,Edificio“Dr.RodrigoBarragán”,2Piso,Ave.MaderoyGonzalitos
S/N,ColoniaMitrasCentro,Monterrey,N.L.,C.P.64460,Mexico. E-mailaddress:carjaime@hotmail.com(J.C.Jaime-Pérez).
lateclonaldisordersreportedafteruptotenyearsoffollow up.4However,latecomplicationsfollowingHDCY,including lastingneutropeniaandsevere fungalinfections havebeen reportedmainlyinadults,butnosimilarlatecomplications havebeenreportedforpatientswhoreceivedHDCYduring childhood.
Wereport thefirst caseofa boywithSAA treated suc-cessfullywithHDCYwhoafter15yearsdevelopedMDSthat rapidlyevolvedintoacutemyeloidleukemia(AML),andwho was treatedunsuccessfully withahematopoietic stem cell transplant(HSCT).
Case
report
Afive-year-oldboywasdiagnosedin1997withSAAaftera shortclinicalperiodoffatigue,anemicsyndromeand muco-cutaneousbleeding,andfindingsconsistentwithSAAinhis completebloodcount(Table1).1Bonemarrowaspirate(BMA)
and biopsy examinations showed a hypoplastic specimen
http://dx.doi.org/10.1016/j.bjhh.2016.06.003
58
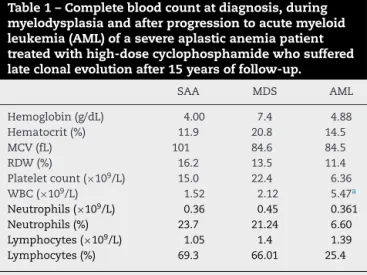
revbrashematolhemoter.2 0 1 7;39(1):57–59Table1–Completebloodcountatdiagnosis,during myelodysplasiaandafterprogressiontoacutemyeloid leukemia(AML)ofasevereaplasticanemiapatient treatedwithhigh-dosecyclophosphamidewhosuffered lateclonalevolutionafter15yearsoffollow-up.
SAA MDS AML
Hemoglobin(g/dL) 4.00 7.4 4.88
Hematocrit(%) 11.9 20.8 14.5
MCV(fL) 101 84.6 84.5
RDW(%) 16.2 13.5 11.4
Plateletcount(×109/L) 15.0 22.4 6.36
WBC(×109/L) 1.52 2.12 5.47a
Neutrophils(×109/L) 0.36 0.45 0.361
Neutrophils(%) 23.7 21.24 6.60
Lymphocytes(×109/L) 1.05 1.4 1.39
Lymphocytes(%) 69.3 66.01 25.4
SAA: severe aplastic anemia; MDS: myelodysplastic syndrome; AML:acute myeloidleukemia;MCV:meancorpuscularvolume; RDW:redcelldistributionwidth;WBC:whitebloodcellcount. a 96%myeloidblasts.
withoutmegakaryocytes,withlessthan15%cellularityand nodysplasticchanges;cytogeneticstudieswerenotavailable inourcenteratdiagnosis.Atestforhypersensitivityof Fan-conianemia(FA)cellstodiepoxybutane,thediepoxybutane (DEB)test,which helpsearlydiagnosis ofFA5 wasnot per-formed.ThepatienthadnoHLA-compatibledonorand,atthat time,therapywithATGplusCSwasnotavailableinour coun-try.ThepatientwaspartofagroupoffivechildrenwithSAA treated withHDCY under these circumstances; the results ofthat trial havebeen published.6 Thetreatment protocol consistedofcyclophosphamide(CY)at50mg/kg/day adminis-teredintravenously(IV)over1honfourconsecutivedayswith atotaldoseof200mg/kg,plusgranulocytecolony stimulat-ingfactor(G-CSF)at5g/kg/day,administeredsubcutaneously fromDay +1post-HDCYuntilanabsoluteneutrophilcount (ANC)>1×109/Lwasreached.Thepatientachievedcomplete hematological remission, and remained freeoftransfusion requirements with no infections. No dysplastic or clonal hematologicaldisordersdevelopedover15yearsoffollow-up. FifteenyearsafterHDCY,thispatientpresentedwithsevere anemiaandhewastreatedwithpackedredbloodcell trans-fusions,folicacid(5mg/day)anddanazol(200mgb.i.d.).Three monthslater,classicalfindingsofmyelodysplasiawere doc-umented in peripheral blood and bone marrow, including hypercellularbonemarrowwithasignificantincreaseinthe numberofmicromegakaryocytesandreticulinfibrosisGrade 3.Fivemonthslater,theMDSprogressedtoalpha-naphthyl butyrateesterase-positiveacutemyeloblasticleukemia (AML-M5).Thebonemarrowimmunophenotypedemonstratedcells withCD4,CD11b,CD16,CD36,CD56,CD64,andHLA-DR anti-genicspecificities.
Importanthematologicdataatthetimeofmyelodysplasia andafterAMLareshowninTable1.
StandardAMLchemotherapywasstarted,including cytara-bineforsevendaysplusmitoxantroneforthreedays.Itwas decidedtoperformhaploidenticalstemcelltransplantation fourteendaysafterthediagnosisofAMLbecauseofthe per-sistence of blasts in the peripheral blood and due to the
backgroundand poorprognosis. Areduced-intensity condi-tioning(RIC)regimenwasadministered45daysaftertheend ofinductionconsistingofcyclophosphamide,fludarabine,and busulfan.HaploidenticalHSCTwasperformedonDay+6of conditioning usinghismotherasdonor. Hepresented neu-tropenia and fever onthe same dayasthe procedure, and antibioticswereadministeredwithnoimprovement. Hema-turiaand melenadevelopedrequiringintensivetransfusion support.Pneumoniaunresponsivetotreatmentwasfollowed bycardiopulmonaryarrest and deathtwodaysafter trans-plant.
Discussion
The treatment of choice for SAA patients who have a
matched sibling donor is HSCT with survival rates of up to90%.1 Front-linematchedunrelateddonorappearstobe a viable option in children, with similar overall survival and event-freesurvivaltotransplantswithmatchedsibling donors. A matched unrelated donor HSCT after failure of IST has proved to be a very good rescue strategy.7 As in our case, mostSAA patients lacka suitable donor and,in the absenceofIST, mortalityis75%.8Currently,aresponse rate of44–80%witha three-tofive-year survivalrate
ran-ging from 81 to 93% for SAA patients immunosuppressed
withATGplusCsAand granulocyte-colonystimulating fac-tor (G-CSF) has been reported.2 AA patients, however, can have apartialresponse,failtorespond,relapse, or remain
dependent of CsA. In order to circumvent these
short-comings, alternative IST, such asalemtuzumab,as well as promisingagents,includingeltrombopag,arecurrentlybeing investigated.
Successful immunosuppressive treatment of SAA with
ATG and CsA has been associated with late clonal disor-ders, including paroxysmal nocturnal hemoglobinuria and MDS.3Highdosecyclophosphamidehasalsobeen success-fully administered inSAA, mostly in adults, withno such late clonaldisorders reportedafteradecade offollow up.4 There is however, evidence for late complications follow-ing HDCY, including lastingneutropenia and severe fungal infections.4 These adverse long-termeventshave notbeen reportedforpatientswhoreceivedHDCYduringchildhood.A recentstudyfocusingontheoutcomesofpediatricpatients withAA foundclonalevolutionand diseaseprogressionto MDS in five patients out of 149 (3%) that had moderate AA.2 Our patienthad a stablecourse withgood qualityof life for15 years aftersuccessful treatmentwith HDCY; he thenevolvedtosevereMDSshortlyfollowedbyAML,treated unsuccessfullywithchemotherapyandfollowedbyamatched relateddonorHSCTthatwascomplicatedbysepsisleadingto death.
revbrashematolhemoter.2 0 1 7;39(1):57–59
59
Acknowledgement
WethankSergioLozano-Rodriguez,M.D.forhisreviewofthe manuscript.
r
e
f
e
r
e
n
c
e
s
1.YoungNS.Currentconceptsinthepathophysiologyand treatmentofaplasticanemia.HematolAmSocHematolEduc Program.2013;2013(1):76–81.
2.ForesterCM,SartainSE,GuoD,HarrisMH,WeinbergOK, FlemingMD,etal.Pediatricaplasticanemiaandrefractory cytopenia:aretrospectiveanalysisassessingoutcomesand histomorphologicpredictors.AmJHematol.2015;90(4):320–6.
3.AfableMG,TiuRV,MaciejewskiJP.Clonalevolutioninaplastic anemia.HematolAmSocHematolEducProgram.
2011;2011(1):90–5.
4.BrodskyRA,ChenAR,DorrD,FuchsEJ,HuffCA,LuznikL,etal. High-dosecyclophosphamideforsevereaplasticanemia: long-termfollow-up.Blood.2010;115(11):2136–41.
5.AuerbachAD.DiagnosisofFanconianemiabydiepoxybutane analysis.CurrProtocHumGenet.2015;85:1–17,8.7.
6.JaimePérezJC,GonzálezLlanoO,GómezAlmaguerD.High dosecyclophosphamideinthetreatmentofsevereaplastic anemiainchildren.AmJHematol.2001;66(1):71.
7.SamarasingheS,StewardC,HiwarkarP,SaifMA,HoughR, WebbD,etal.Excellentoutcomeofmatchedunrelateddonor transplantationinpaediatricaplasticanaemiafollowing failurewithimmunosuppressivetherapy:aUnitedKingdom multicentreretrospectiveexperience.BrJHaematol. 2012;157(3):339–46.
8.BacigalupoA,BrandR,OnetoR,BrunoB,SodéG,PasswegJ, etal.Treatmentofacquiredsevereaplasticanemia:bone marrowtransplantationcomparedwithimmunosuppressive therapy–theEuropeangroupforbloodandmarrow