rev bras hematol hemoter. 2017;39(1):60–62
w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Case
Report
Detection
of
Human
Adenovirus
(species-C,
-D
and
-F)
in
an
allogeneic
stem
cell
transplantation
recipient:
a
case
report
Hugo
César
Pereira
Santos
a,
Francielly
Pinheiro
da
Silva
Borges
a,
Adriano
de
Moraes
Arantes
b,
Menira
Souza
a,∗aUniversidadeFederaldeGoiás(UFG),Goiânia,GO,Brazil
bHospitalAraújoJorge,Associac¸ãodeCombateaoCânceremGoiás(ACCG),Goiânia,GO,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received12July2016 Accepted21September2016 Availableonline19October2016
HumanAdenovirus (HAdV) commonlycauses mild clinical symptomsin immunocompetentpatients. In immunocom-promisedindividuals,suchaspatientssubmittedtoallogeneic stemcell transplantation(aSCT),HAdVinfection cancause prolonged and disseminated disease, resulting in a worse prognosisforthepatientorevendeath.1
A57-year-oldBrazilianmanwasdiagnosedwithchronic myeloid leukemia and during treatment with imatinib he developedresistancesecondary toaT315I mutationinthe BCR-ABLkinasedomain.Ahumanleukocyteantigen (HLA)-identicalsiblingdonorwasavailable,andthepatientwas sub-mittedtostemcelltransplantationinOctober2012with non-myeloablativeconditioningbasedonfludarabine(150mg/m2) and busulfan (16mg/kg). Trimethoprim/sulfamethoxazole, acyclovir and fluconazolewere given as antimicrobial pro-phylaxis.Graft-versus-hostdisease (GVHD)prophylaxiswas achievedwithcyclosporine(3mg/kg)fromDay1priorto trans-plant(D−1)and ashortcourseofmethotrexate(15mg/m2 onD+1and10mg/m2onD+3,D+6,D+11post-transplant).
∗ Correspondingauthorat:InstitutodePatologiaTropical,UniversidadeFederaldeGoiás,Rua235,s/n,sala420,SetorUniversitário,
74605050Goiânia,GO,Brazil.
E-mailaddress:menirasouza@gmail.com(M.Souza).
Cellsourcewasnon-stimulatedbonemarrowand2.8×108/kg of total nucleated cells without ABO incompatibility were infused.
ThisstudywasapprovedbytheResearchEthics Commit-tee oftheHospitalAraújo Jorge/Associac¸ão deCombate ao CânceremGoiás(ACCG:protocol#108.396).Thepatientsigned a consentformforhisclinical samples(feces and sera)to bemonitoredforgastroentericviruses.Thefirstsamplewas collectedonD+1,andsubsequentlysampleswereobtained weeklyuntilpatientdischarge.Sampleswerethencollected duringoutpatientvisits.Sampleswereprocessedusing com-mercialkits(QIAampStoolMiniKitandQIAampMinEluteSpin Kit,QIAGEN,Freigburg,Germanyforstoolandserum, respec-tively),followingthemanufacturer’sinstructions.
ToscreenforHAdV,samplesweresubjectedto quantita-tivepolymerasechainreaction(PCR–Taqman)aspreviously described withadaptations.2,3 Briefly,pureand1:10diluted
DNAwasaddedtoa25Lmixcontaining1×ofMasterMix (appliedBiosystems),0.9Mofeachprimerand0.225Mof
http://dx.doi.org/10.1016/j.bjhh.2016.09.006
revbrashematolhemoter.2017;39(1):60–62
61
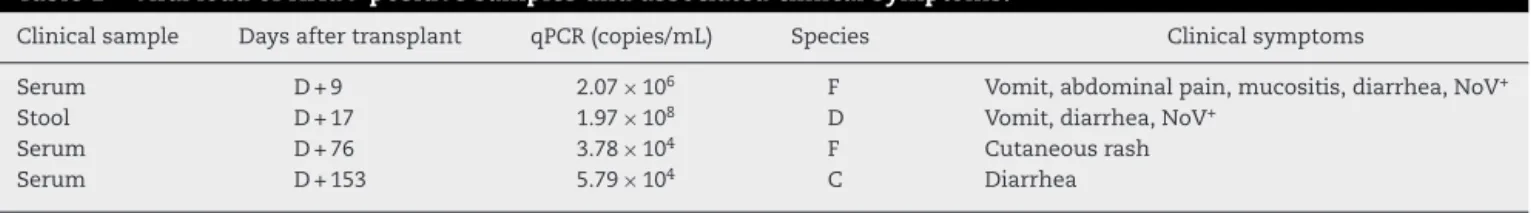
Table1–ViralloadofHAdV-positivesamplesandassociatedclinicalsymptoms.
Clinicalsample Daysaftertransplant qPCR(copies/mL) Species Clinicalsymptoms
Serum D+9 2.07×106 F Vomit,abdominalpain,mucositis,diarrhea,NoV+
Stool D+17 1.97×108 D Vomit,diarrhea,NoV+
Serum D+76 3.78×104 F Cutaneousrash
Serum D+153 5.79×104 C Diarrhea
qPCR:quantitativepolymerasechainreaction;NoV:norovirus.
GradeIIgraft-versus-hostdiseaseoftheskinandliverwaspresentfromD+41toD+76.
TaqmanprobeslabeledwithFAM-TAMRA,targetingaregion of 72 base pairs. The cycling program was the following: 50◦C for 1min, 95◦C for 10min, followed by 45 cycles of
95◦C for 15s and 60◦C for 1min. Samples were run with
standardcurves (R>0.98)constructedusing serialdilutions (10−2to108)ofthepBR322plasmidcontainingtheHAdVhexon
gene.Resultsareexpressedasgenomiccopiespermilliliter (copies/mL).
HAdV-positive samples were alsosubjected to genomic sequencinginanautomaticsequencer(DNAABIPRISM3130, AppliedBiosystems),usingpurifiednested-PCRproducts,in duplicates,amplifiedbyprimerstargetingaconservedregion ofthehexongene,asdescribedbyPuigetal.4
ThesamplesfromdaysD+1,D+3andD+6werenegative, andthepatientremainedasymptomaticuntilD+9,whenhe presentednausea,vomit,abdominalpain,feveranddiarrhea. Atthis time, the patient alsopresented severe leucopenia (<1000cells/mm3)and lymphopenia(<300cells/mm3).HAdV speciesFwasdetectedinserum(GenBankaccessionnumber KP894106)withaviralloadof2.07×106copies/mL(Table1). ThissamplewasalsopositivefornorovirusGI.3identifiedby reversetranscription PCR.5 Thepatientstillpresentedwith
diarrhea,leucopeniaandlymphopeniaonD+17,andhisfecal sampletestedpositiveforHAdVspeciesD(GenBankaccession numberKP894104)withaviralload of1.97×108copies/mL. ThissamplewasalsopositivefornorovirusGI.3.
On D+21, the patient was discharged. A fecal sample obtainedduring an outpatient visit on D+27 was positive forHAdV(6.94×1010copies/mL)and fornorovirus(GI.3); at the time the patient presented with diarrhea, abdominal painandvomit.ThepatientreturnedonD+41withaskin rash,vomit,fever andabdominal pain;a clinical examina-tionrevealedanacuteskinrash(<25%ofbodysurfacearea) and hyperbilirubinemia (3.14mg/dL)compatible withacute gradeIIGVHD. Afterreadmission,the patient was submit-tedtointravenousrehydrationandimmunosuppressionwith cyclosporine(3mg/kg)andmetilprednisolone(2mg/kg).After fivedays,thepatient’sclinicalstatushadimprovedandthe symptoms ceased. He was discharged with a prescription oforalcyclosporine(10mg/kg)andprednisone(1mg/kg).At thistime,sampleswerenegativeforHAdV,but positivefor norovirusGI.3.
OnD+76,thepatientstillpresentedwithacuteGVHD,and showedpositivityforHAdVspeciesF(3.78×104copies/mL)in aserumsample.PhylogeneticanalysisrevealedthattheHAdV sequencewasidenticaltothesequencefoundinthesample obtainedonD+9.Progressive reductionofprednisonewas madeandinterruptedonD+108.Cyclosporinewasreduced andremovedonD+180.
On D+153,the patientwas sufferingfromdiarrhea and presentedpositivityforHAdVspeciesCinserum(GenBank accession number KP894102; 5.79×104copies/mL). Subse-quentsampleswerenegativeforHAdVandnorovirus,andthe patientremainedasymptomaticandwithoutsignsofGVHD. On D+180afteraSCThewasincomplete remissionand a studyofshorttandemrepeats(STR)showedchimerism com-patible with100% ofdonorcells andquantitative BCR-ABL transcriptnegativeinperipheralblood.Morethanthreeyears aftertransplant,thepatientremainsalivewithout immuno-suppression.
Discussion
HAdVinfectionmayresultinaworseprognosisforpatients submittedtoaSCT.6Insomecases,HAdVinfectionisclinically
diagnosedasGVHD,andtheuseofimmunosuppressive ther-apyinthesecasescouldfurtherimpairthepatient’sclinical condition.7Inthiscase,threedifferentspeciesofHAdVwere
identifiedinsamplesfromthesamepatientuptoD+153. During the periodinwhichthe patientwas positivefor HAdVspeciesFandD,eventhoughhepresentedsymptoms that are characteristic ofenteric HAdV infection (diarrhea, abdominalpainandvomit),hewasalsopositivefornorovirus, whichmayhaveinfluencedandperhapsintensifiedthe symp-toms.
After asequence ofnegative sera samples, onesample waspositiveforHAdVspeciesFonD+76thathadan iden-tical sequence to the sample detected on D+9, this time with lower viral load, suggesting that viremia was inter-mittent.Atthis time, wewere unabletodifferentiate viral reactivationfrompersistentacuteGVHD.Wehypothesizethat theimmunosuppressivetherapyimpairedviralclearancedue totheearlytransplantationphaseassociatedwith supposi-tional delayinT-cellimmunereconstitution. Thiscouldbe owing to inefficient thymopoiesis, probably caused by the intensiveconditioningregimen,acuteGVHD,useof cortico-steroidsandtherecipient’sage.
62
revbrashematolhemoter.2017;39(1):60–62transplanted patientswithhigh viral loads,indicatingthat infectiondoesnotalwaysnecessarilycausesymptoms.8
ThiscasereportshowsthatdistinctHAdVspeciesmaybe presentinthenosocomialenvironment,anddespitehaving strictinfectionandtransmissioncontrolmeasures,theyare notalwayssufficienttoavoidviralcirculationinthehospital, aspreviouslyobserved.9
Thispatient was the first in aseries of patients moni-toredforHAdVinfection(unpublisheddata).Inthepatients thatfollowed,HAdVspeciesFandC,withidenticalgenomic sequencesasfoundinthesamplesofthispatient,werealso detected.Therefore,wespeculatethathewaseitherthefirst patienttobecomeHAdV-positiveinaseriesofcases,orthat hemayhavebeentheoriginalcarrierwhointroducedthese virusesintothe hospital,whichremainedcirculatingforat leasttwomoreyears(dataunpublished).Itisalsopossiblethat HAdV-positivityisaresultofadenovirusreactivationfroma previouslylatentinfection.10
The results highlight that HAdV can be present in the nosocomialenvironment,withthepossibilityofitremaining infectious for months after its first introduction, and that existing infectionpreventionprotocols are notsufficientto preventviruscirculation.InBrazil,patientsundergoingaSCT arenotmonitoredforHAdVinfection,butourdata demon-stratetheimportanceofmonitoringclinicalsamplesofthese patientsinordertoprovideanappropriatetreatmentwhen theinfectionandtheclinicalsymptomsarepresent.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
Theauthors wish tothank Fundac¸ãode Apoio aPesquisa emGoiás(FAPEG)andConselhoNacionaldeDesenvolvimento CientíficoeTecnológico(CNPq)forfinancialsupport.
r
e
f
e
r
e
n
c
e
s
1.WoldWSM,IsonMG.Adenoviruses.In:KnipeDM,HowleyPM,
editors.Fieldsvirology.6thed.Philadelphia:Lippincott
Williams&Wilkins;2013.p.1732–67.
2.HernrothBE,Conden-HanssonAC,Rehnstam-HolmAS,
GiroresR,AllardAK.Environmentalfactorsinfluencing
humanviralpathogensandtheirpotentialindicator
organismsinthebluemussel,Mytilusedulis:thefirst
Scandinavianreport.ApplEnvironMicrobiol.
2002;68(9):4523–33.
3.MellouK,SideroglouT,Potamiti-KomiM,KokkinosP,ZirosP,
GeorgakopoulouT,etal.Epidemiologicalinvestigationoftwo
parallelgastroenteritisoutbreaksinschoolsettings.BMC
PublicHealth.2013;13:241.
4.PuigM,JofreJ,LucenaF,AllardA,WadellG,GironesR.
Detectionofadenovirusesandenterovirusesinpolluted
watersbynestedPCRamplification.ApplEnvironMicrobiol.
1994;60(8):2963–70.
5.LemesLG,CorrêaTS,FiaccadoriFS,CardosoDd,ArantesAde
M,SouzaKM,etal.ProspectivestudyonNorovirusinfection
amongallogeneicstemcelltransplantrecipients:prolonged
viralexcretionandviralRNAintheblood.JClinVirol.
2014;61(3):329–33.
6.HierholzerJC.Adenovirusesintheimmunocompromised
host.ClinMicrobiolRev.1992;5(3):262–74.
7.MarrKA.Delayedopportunisticinfectionsinhematopoietic
stemcelltransplantationpatients:asurmountablechallenge.
Hematology:AmSocHematolEducProgram.
2012;2012:265–70.
8.ClaasEC,SchilhamMW,deBrouwerCS,HubacekP,Echavarria
M,LankesterAC,etal.Internallycontrolledreal-timePCR
monitoringofadenovirusDNAloadinserumorplasmaof
transplantrecipients.JClinMicrobiol.2005;43(4):1738–44.
9.GanimeAC,Carvalho-CostaFA,SantosM,CostaFilhoR,Leite
JP,MiagostovichMP.Viabilityofhumanadenovirusfrom
hospitalfomites.JMedVirol.2014;86(12):2065–9.
10.HiwarkarP,GasparHB,GilmourK,JaganiM,ChiesaR,
Bennett-ReesN,etal.Impactofviralreactivationsintheera
ofpre-emptiveantiviraldrugtherapyfollowingallogeneic
haematopoieticSCTinpaediatricrecipients.BoneMarrow