www.jped.com.br
ORIGINAL
ARTICLE
Lactobacillus
reuteri
DSM
17938
shortens
acute
infectious
diarrhea
in
a
pediatric
outpatient
setting
夽
Ener
Cagri
Dinleyici
a,∗,
Nazan
Dalgic
b,
Sirin
Guven
c,
Ozge
Metin
d,
Olcay
Yasa
e,
Zafer
Kurugol
f,
Ozden
Turel
g,
Gonul
Tanir
d,
Ahmet
Sami
Yazar
c,
Vefik
Arica
h,
Mesut
Sancar
i,
Adem
Karbuz
h,
Makbule
Eren
j,
Metehan
Ozen
h,
Ates
Kara
i,
Yvan
Vandenplas
kaFacultyofMedicine,DepartmentofPediatrics,PediatricIntensiveCareandInfectiousDiseaseUnit,EskisehirOsmangazi
University,Eskisehir,Turkey
bDivisionofPediatricInfectiousDiseases,SisliEtfalTrainingandResearchHospital,Istanbul,Turkey cDepartmentofPediatrics,UmraniyeEducation&ResearchHospital,Istanbul,Turkey
dPediatricInfectiousDiseaseUnit,Dr.SamiUlusResearchandTrainingHospitalofWomen’sandChildren’sHealthandDiseases,
Ankara,Turkey
eDepartmentofPediatrics,GoztepeTrainingandResearchHospital,SBIstanbulMedeniyetUniversity,Istanbul,Turkey fDepartmentofPediatrics,FacultyofMedicine,EgeUniversity, ˙Izmir,Turkey
gDepartmentofPediatricInfectiousDiseaseUnit,FacultyofMedicine,BezmialemVakifUniversity,Istanbul,Turkey hDepartmentofPediatrics,OkmeydaniEducationandResearchHospital,Istanbul,Turkey
iFacultyofPharmacy,ClinicalPharmacyDepartment,MarmaraUniversity,Istanbul,Turkey jFacultyofMedicine,PediatricInfectiousDiseaseUnit,HacettepeUniversity,Ankara,Turkey kDepartmentofPediatrics,UZBrussel,VrijeUniversiteitBrussel,Brussels,Belgium
Received1August2014;accepted23October2014 Availableonline16May2015
KEYWORDS Lactobacillusreuteri DSM17938;
Diarrhea; Children; Probiotic; Ambulatorycare
Abstract
Objective: TworandomizedcontrolledclinicaltrialshaveshownthatLactobacillus(L)reuteri DSM17938reducesthedurationofdiarrheainchildren hospitalizedduetoacuteinfectious diarrhea.ThiswasthefirsttrialevaluatingtheefficacyofL.reuteriDSM17938inoutpatient childrenwithacuteinfectiousdiarrhea.
Methods: Thiswasamulticenter,randomized,single-blinded,casecontrolclinicaltrialin chil-drenwithacutewaterydiarrhea.A totalof64childrenwho presentedatoutpatientclinics wereenrolled.Theprobioticgroupreceived1×108CFUL.reuteriDSM17938forfivedaysin additiontooralrehydrationsolution(ORS)andthesecondgroupwastreatedwithORSonly.
夽
Pleasecitethisarticleas:DinleyiciEC,DalgicN,GuvenS,MetinO,YasaO,KurugolZ,etal.LactobacillusreuteriDSM17938shortens acuteinfectiousdiarrheainapediatricoutpatientsetting.JPediatr(RioJ).2015;91:392---6.
∗Correspondingauthor.
E-mail:timboothtr@yahoo.com(E.C.Dinleyici).
http://dx.doi.org/10.1016/j.jped.2014.10.009
Theprimaryendpointwasthedurationofdiarrhea(inhours).Thesecondaryendpointwasthe numberofchildrenwithdiarrheaateachdayofthefivedaysofintervention.Adverseevents werealsorecorded.
Results: The mean duration of diarrhea was significantly reduced in the L. reuteri group compared tothe controlgroup (approximately 15h, 60.4±24.5h [95%CI: 51.0---69.7h]vs. 74.3±15.3h[95%CI:68.7---79.9h], p<0.05).The percentage ofchildrenwith diarrheawas lowerintheL.reuterigroup(13/29;44.8%)after48hthanthecontrolgroup(27/31;87%;RR: 0.51;95%CI:0.34---0.79,p<0.01).Fromthe72ndhourofinterventiononwards,therewasno differencebetween thetwo groupsinthepercentageofchildrenwithdiarrhea. Noadverse effectsrelatedtoL.reuteriwerenoted.
Conclusion: L.reuteriDSM17938iseffective,safe,andwell-toleratedinoutpatientchildren withacuteinfectiousdiarrhea.
©2015SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
PALAVRAS-CHAVE Lactobacillusreuteri DSM17938;
Diarreia; Crianc¸as; Probióticos;
Cuidadoambulatorial
OLactobacillusreuteriDSM17938reduzadiarreiainfecciosaagudanocuidado pediátricoambulatorial
Resumo
Objetivo: Dois ensaiosclínicos randomizadoscontrolados demonstraramqueoLactobacillus (L)reuteriDSM17938reduzadurac¸ãodediarreiaemcrianc¸ashospitalizadasdevidoadiarreia infecciosaaguda.EsteéoprimeiroensaioqueavaliaaeficáciadoL.reuteriDSM17938em crianc¸ascomdiarreiainfecciosaagudanoambulatório.
Métodos: Estefoiumensaioclínicomulticêntrico,randomizado,únicocego,comgrupos par-alelos econtroladoem crianc¸as comdiarreia aguda. Umtotalde64 crianc¸as internadasna clínica ambulatorial foraminscritas.Ogrupo probióticorecebeu 1×108CFU L. reuteriDSM 17938porcincodias,alémdeumasoluc¸ãodereidratac¸ãooral(SRO),eosegundogrupofoi tratadoapenascomSRO.Odesfechoprincipalfoiadurac¸ãodadiarreia(emhoras).Odesfecho secundáriofoionúmerodecrianc¸ascomdiarreiaemcadaumdoscincodiasdaintervenc¸ão. Oseventosadversostambémforamregistrados.
Resultados: Adurac¸ãomédiadadiarreiafoisignificativamentereduzidanogrupoL.reuteri emcomparac¸ãoaogrupodecontrole(aproximadamente15horas;60,4±24,5horas[51,0---69, 7horas,ICde95%]emcomparac¸ãoa74,3±15,3horas[68,7---79,9horas,ICde95%],p<0,05). Opercentualdecrianc¸ascomdiarreiafoimenornogrupoL.reuteri(13/29;44,8%)após48 horasquenogrupodecontrole(27/31;87%)(RR:0,51;0,34---0,79;ICde95%,<0,01).Apartir da72ahoradeintervenc¸ão,nãohavianenhumadiferenc¸aentreosdoisgruposnopercentual
decrianc¸ascomdiarreia.Nenhumefeitoadversocomrelac¸ãoaoL.reuterifoiobservado. Conclusão: OL.reuteriDSM17938éeficaz,seguroebemtoleradoporcrianc¸ascomdiarreia infecciosaagudanoambulatório.
©2015SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos reservados.
Introduction
Diarrhea remains an important cause of morbidity and mortality in children worldwide.1 The European Society
of Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) and the European Society of Paediatric Infec-tious Diseases (ESPID) together published evidence-based guidelinesonthemanagementofacutegastroenteritis,and confirmedthatrehydrationisthekeytreatment.The guide-lines alsostated that selected probiotics mayreduce the duration and intensity of symptoms and can be used as an adjuvant to oral rehydration solution (ORS).2 Current
evidence also indicates that probiotic effects are strain-specific.3 Various strains of Lactobacillus reuteri species
havebeen studiedin acutediarrheaand havebeen found tobebeneficial. L. reuteriDSM 17938 is anew probiotic strainwithremovedtransferableresistancetraitsfor tetra-cycline and lincomycin from the original L. reuteri ATCC 55730strain.4IthasbeenpreviouslyreportedthatL.reuteri
DSM17938reducedthedurationofdiarrheaandthelength of hospital stay in children requiring hospitalization due toacuteinfectiousdiarrhea.5Arecentmeta-analysisofL.
reuteriDSM 17938 in children with acuteinfectious diar-rheashowed reduced durationof diarrhea and concluded thatoutpatientdataandcountry-specificcost-effectiveness analysesareneeded.6This study is thefirstreport ofthe
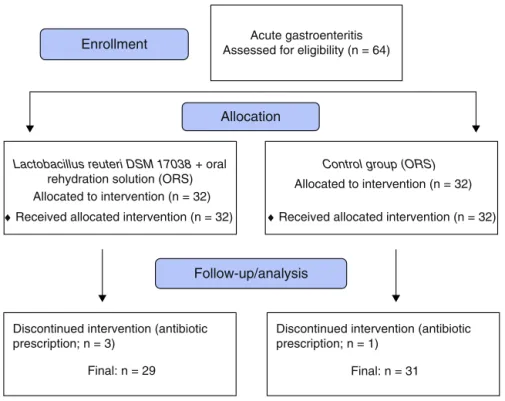
Acute gastroenteritis Assessed for eligibility (n = 64)
Discontinued intervention (antibiotic prescription; n = 3)
Final: n = 29
Lactobacillus reuteri DSM 17038 + oral rehydration solution (ORS) Allocated to intervention (n = 32)
♦ Received allocated intervention (n = 32) Allocation
Follow-up/analysis Enrollment
Discontinued intervention (antibiotic prescription; n = 1)
Final: n = 31
Control group (ORS)
Allocated to intervention (n = 32)
♦ Received allocated intervention (n = 32)
Figure1 Flowdiagramofthestudygroup.
Patient
and
methods
This was a multicenter, randomized, single-blinded, case control clinical trial in children of both genders, aged between3 and60 months,withacute infectious diarrhea (definedasthe passageof threeor more loose or watery stoolsperday) lasting12---72hbefore presentationat the outpatientclinic.Themaininvestigator(ECD)didnotenroll childrenandwasblindedtothetreatmentofthepatients. Approval for the study was granted by the Local Ethics Committee and an informed consent was obtained from the parents of the children. This study was registered at
www.clinicaltrials.gov(NCT01927094).
Children who presented at the outpatient clinic with acuteinfectious diarrhea,andwhowerefollowedupwith ambulatorycarewereenrolledinthestudy.Exclusion crite-ria were need for hospitalization, use of antibiotics or probioticsforonemonthpriortoanewepisodeofdiarrhea, severemalnutrition,or severechronic underlyingdisease, includingimmunocompromisingconditions.
Allchildrenwererandomlyassignedtotheprobioticor control group using a computer-generated randomization list. Block randomization was applied with a computer-generated random number list by the main investigator (ECD), who did not enroll any patients. The first group receivedthefivedropscontaining1×108CFUL.reuteriDSM
17938(BioGaia®,Stockholm,Sweden)forfivedays,in
addi-tiontoORS. The second groupreceivedORS only(control group).The probiotics preparations wereprovidedby the distributioncompany(Eczacibasi)in Turkey.Hypo-osmolar ORS(glucose20g;sodium60mmol/L;potassium20mmol/L; bicarbonate 30mmol/L) was used. The primary endpoint wasthedurationofdiarrhea(inhours).Thesecondary end-pointwasthenumberofchildrenwithdiarrheaateachday ofthefivedaysoftheintervention.Adverseeventswerealso
recorded.Thedurationofdiarrheawasdefinedasthetime inhoursfromadmissionuntilcessationofdiarrhea,whichin turnwasdefinedasthefirstnormalstoolaccordingtothe Bristolscore(ascoreof<5wasconsideredasnormalization ofthestools).
The sample size needed was calculated based on the meandurationofdiarrheaandstandarddeviation(SD)from previoussimilarstudies.Withtheassumptionofmean differ-enceondurationofdiarrheaofoneday(24h)betweenthe treatment and control group, theauthors calculated that asampleof64childrenwouldberequiredforthestudyto have80%powerwithasignificancelevel=0.05andsigma=2 (two tailed test). Statistical analysiswasperformed using SPSSsoftware,version 16.0(SPSSInc.,IL, USA). Variables weretestedfornormaldistributionandcomparedusingthe Mann---WhitneyU-testandt-test(formeandifference)and 2 or Fisher’s exact tests, asappropriate. MedCalc® pro-gram (Microsoft Partner Network, USA) was performed to calculaterelativerisk(RR).Statisticalsignificancewasset atp<0.05.
Results
Thestudyincluded64children.Afterexclusionofthree chil-drenfromtheL.reuterigroupandonechildfromthecontrol group due to antibiotic prescription post-randomization (Fig.1),atotalof60remainedforevaluation;29(20male,9 female)intheL.reuterigroupand31(22male,ninefemale) inthecontrolgroup.Thedemographicfindings,mean dura-tion ofdiarrhea beforeintervention andmean number of stoolsat24hpriortoinclusionaresummarizedinTable1.
Table1 Demographicandclinicalfindingsofthestudygroups.
LactobacillusreuteriDSM17938(n=29) Controlgroup(n=31)
Age(months) 27.9±18.2 22.6±14.4
Gender 20boys,9girls 22boys,9girls
Meannumberofstoolsduringthe24hpriortoinclusion 7.13±3.8 6.1±0.9 Meandurationofdiarrheabeforeintervention(hours) 18.1±6.5 20.1±8.7
Valuesexpressedasmean±SD.
Table2 Durationofdiarrheainstudygroups.
Lactobacillus reuteriDSM17938 (n=29)
Controlgroup (n=31)
Durationof diarrhea(hours)
60.4±24.5a 74.3±15.3
Valuesareexpressedasmean±SD(95%CI).
a p=0.01;L.reuterivs.controlgroup.
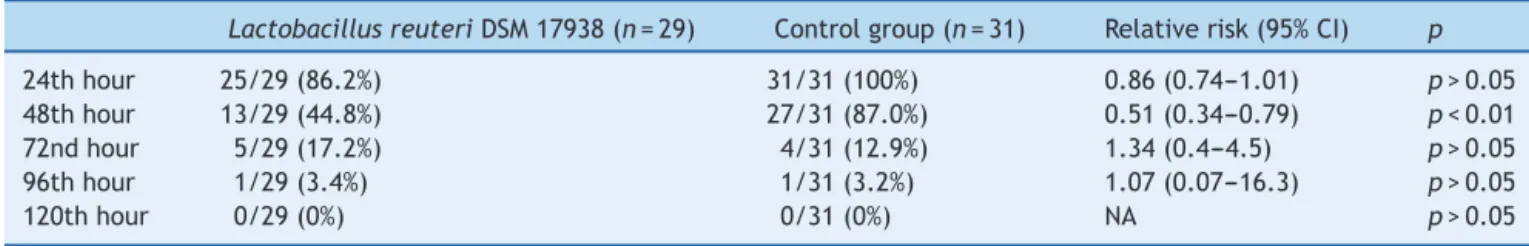
Atthe48thhourofthestudy,45%ofthechildren receiv-ing L.reuteri DSM 17938 had watery diarrhea,while this wasstillthecasein87%ofthechildreninthecontrolgroup (13/29;44.8%vs.27/31;87%;RR:0.51;95%CI:0.34---0.79; p<0.01).Fromthe72ndhouroftheintervention,the per-centageof childrenwithout diarrheawassimilarbetween thegroups(Table3).NoadverseeffectsrelatedtoL.reuteri
DSM17938wereobserved.
Discussion
TheresultsofthepresentstudyshowedthatORSin combi-nationwithfivedaysofL.reuteriDSM17938 reducedthe durationofacuteinfectiousdiarrheatoapproximately15h inchildrenagedbetween3and60months.Theeffectwas mainlyobservedafter48hofintervention,when55%ofthe childrenintheinterventiongroupwerediarrhea-free,while thiswasthecaseinonly13%inthecontrolgroup.Ina previ-ousstudy,performedbythesamestudyteamandwiththe samedesign,exceptforthefactthatthestudywasapplied to hospitalized children, 127 children aged 3---60 months wereenrolled.Inhospitalizedchildren,theadministration ofL.reuteriDSM17938reducedthedurationofdiarrhea sig-nificantlytoapproximately33h.5Theeffect(percentageof
childrenwithoutdiarrhea)ofL.reuteriDSM17938startedto beobservedafter24hofinterventionandwasgreatestafter 48and72h.Itwasobservedalsothatthemeanlengthof hos-pitalstaywasshortenedbymorethan24hintheL.reuteri
DSM17938group.5Francavillaetal.7performeda
random-izeddouble-blinded,placebo-controlledclinicaltrialon74 children aged6---36 months with acutediarrhea, random-izedtoreceive L.reuteriDSM17938 orplacebo forseven days,inthreehospitalsinSouthernItaly.Comparedwiththe placebogroup,theL.reuteriDSM17938grouppresenteda significantreductioninthedurationofdiarrhea,intherisk ofwaterydiarrheaonday2 andday 3,andin therisk of relapseofdiarrhea,althoughnoreductionoftheduration ofhospitalizationwasobserved.7Szajewska etal.6pooled
thedatafromthesetwoRCTsofhospitalizedchildren, con-firmingthatL.reuteriDSM17938significantlyreducedthe durationof diarrheato approximately 32h and increased thechanceofcureonday3.6Twoindependentstudies,one
inIndonesiaandoneinMexico,demonstratedthatthe pro-phylacticuseofL.reuteriDSM17938reducesthefrequency anddurationofdiarrhealepisodes,8,9althoughtheirclinical
relevancecan bequestioned.10 L. reuteri DSM 17938 was
welltolerated,andnoassociatedadverseeventshavebeen reportedinanytrial.6
Thecurrentstudyhadsomelimitations,asitwasnota double-blinded,placebo-controlledclinicaltrial. Toaccess stool consistency, the Bristol Stool Form Scale was used, which has a limited validation for the youngest children, althoughitoffersamoreobjectiveformofassessingstool consistencyratherthan justrelyingonthe perceptionsof caregivers.11 Thedurationofdiarrheawasusedasthe
pri-maryendpoint, whichisnotconsideredoptimal.However, thereis a major reluctance of caregivers and healthcare providers to conduct the cumbersome stool collection. The question of generalization of findings in clinical tri-als,specificallyoninfectiousgastroenteritisandprobiotics, is challenging. However,these findingscan be considered asgeneralized,sinceworldwideviralgastroenteritisisthe mostcommoncause.Moreover,asamulti-centerstudy,the participantscame fromurban andruralregions, including economicallydeveloped and poorareas. Therefore,these findingsarelikelytoallowforgeneralization.
The WorkingGroup onProbiotics ofthe European Soci-etyforPediatricGastroenterology,HepatologyandNutrition
Table3 Percentageofchildrenwithdiarrheainstudygroups.
LactobacillusreuteriDSM17938(n=29) Controlgroup(n=31) Relativerisk(95%CI) p
24thhour 25/29(86.2%) 31/31(100%) 0.86(0.74---1.01) p>0.05
48thhour 13/29(44.8%) 27/31(87.0%) 0.51(0.34---0.79) p<0.01
72ndhour 5/29(17.2%) 4/31(12.9%) 1.34(0.4---4.5) p>0.05
96thhour 1/29(3.4%) 1/31(3.2%) 1.07(0.07---16.3) p>0.05
(ESPGHAN)concludedthattheuseofL.reuteriDSM17938 shouldbeconsideredinthemanagementofacute gastroen-teritisasanadjunctinterventiontoORS.3Probioticsmaybe
ofhelpforthemanagementofacutediarrhea.However,the effectisstrain-specific,andefficacyneedstobeprovenin differentsettings,suchashospitaloroutpatientssettings. TheresultsofthepresentstudyhaveshownthatL.reuteri
DSM17938asanadjuncttoORStherapyisefficaciousinthe treatmentofacutediarrhea,reducingthedurationofthe diseaseinanoutpatientsetting.
Conflicts
of
interest
E.C.Dinleyiciisspeakerbureauandadvisoryboardmember ofBiocodex.M.OzenisspeakerbureauofPfizerConsumer Health.Y. Vandenplasis aconsultantforBiocodex,United Pharmaceuticals.Otherauthorsdeclarenoconflictsof inter-est.
References
1.WalkerCL,RudanI,LiuL,NairH,TheodoratouE,BhuttaZA, et al.Global burdenofchildhood pneumoniaanddiarrhoea. Lancet.2013;381:1405---16.
2.Guarino A, Albano F, Ashkenazi S, Gendrel D, Hoekstra JH, Shamir R, et al. European Society for Paediatric Gas-troenterology, Hepatology, and Nutrition/European Society for Paediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: executive summary. J Pediatr Gastroenterol Nutr. 2008;46:619---21.
3.Szajewska H, Guarino A, Hojsak I, Indrio F, Kolacek S, ShamirR, et al. Useof probioticsfor management ofacute
gastroenteritis:apositionpaperbytheESPGHANWorkingGroup for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2014;58:531---9.
4.Urba´nskaM,SzajewskaH.TheefficacyofLactobacillusreuteri DSM17938ininfantsandchildren:areviewofthecurrent evi-dence.EurJPediatr.2014;173:1327---37.
5.Dinleyici EC, Vandenplas Y. PROBAGE Study Group Lacto-bacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children. Acta Paediatr. 2014;103:e300-e305.
6.SzajewskaH,Urba´nskaM,ChmielewskaA,WeizmanZ,Shamir R.Meta-analysis.LactobacillusreuteristrainDSM17938(and theoriginalstrainATCC55730)fortreatingacutegastroenteritis inchildren.BenefMicrobes.2014;5:285---93.
7.Francavilla R, Polimeno L, Demichina A, Maurogiovanni G, PrincipiB, ScaccianoceG, et al. Lactobacillusreuteristrain combination in Helicobacterpylori infection: a randomized, double-blind,placebo-controlled study.JClin Gastroenterol. 2014;48:407---13.
8.AgustinaR,KokFJ,vandeRestO,FahmidaU,FirmansyahA, LukitoW,etal.Randomizedtrialofprobioticsandcalciumon diarrheaandrespiratorytractinfectionsinIndonesianchildren. Pediatrics.2012;129:e1155---64.
9.Gutierrez-Castrellon P, Lopez-Velazquez G, Diaz-Garcia L, Jimenez-Gutierrez C, Mancilla-Ramirez J, Estevez-Jimenez J, et al. Diarrhea in preschool children and Lactobacillus reuteri: a randomizedcontrolled trial. Pediatrics.2014;133: e904---9.
10.VandenplasY.Lactobacillusreuteri isaneffectiveoptionfor the prevention of diarrhoea in preschool children but may notbecost-effectiveinallsettings.EvidBasedMed.2014;19: 212.