w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Effects
of
(
−
)-6,6
′
-dinitrohinokinin
on
adult
worms
of
Schistosoma
mansoni:
a
proteomic
analyses
Lizandra
G.
Magalhães
a,∗,
Thais
C.
Lima
a,
Renato
G.
de
Paula
b,
Enyara
R.
Morais
c,
Daniela
P.
Aguiar
a,
Luiz
G.
Gardinassi
b,
Gustavo
R.
Garcia
b,
Rosangela
S.
Laurentiz
d,
Vanderlei
Rodrigues
b,
Jairo
K.
Bastos
e,
Ademar
A.S.
Filho
f,
Ana
P.
Yatsuda
e,
Wilson
R.
Cunha
a,
Márcio
L.A.
Silva
aaGrupodePesquisaemCiênciasExataseTecnológicas,UniversidadedeFranca,Franca,SP,Brazil bFaculdadedeMedicinadeRibeirãoPreto,UniversidadedeSãoPaulo,RibeirãoPreto,SP,Brazil cInstitutodeGenéticaeBioquímica,UniversidadeFederaldeUberlândia,PatosdeMinas,MG,Brazil dFaculdadedeEngenharia,UniversidadedoEstadodeSãoPaulo,IlhaSolteira,SP,Brazil
eFaculdadedeCiênciasFarmacêuticas,UniversidadedeSãoPaulo,RibeirãoPreto,SP,Brazil fFaculdadedeCiênciasFarmacêuticas,UniversidadedeJuizdeFora,JuizdeFora,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received18September2015 Accepted3February2016 Availableonline3March2016
Keywords:
(−)-6;6′-dinitrohinokinin
Lignan
Massspectrometry Proteome
Schistosomamansoni
Two-dimensionalgelelectrophoresis
a
b
s
t
r
a
c
t
Schistosomiasis,achronicdiseasethataffectsmillionpeopleworldwide,iscaused bytrematode flukesofthegenusSchistosoma.Thelackofananti-schistosomiasisvaccineandmassive monother-apywithpraziquantel reinforces theneed forsearch and developmentof new therapeutic drugs. Recently,wedemonstratedthattheessentialoilofPipercubebaL.,Piperaceae,andtheirderivative diben-zylbutyrolactolic(−)-6,6′-dinitrohinokinin,presentsinvitroandinvivoactivitiesagainstSchistosoma
mansoni.Here,weidentifiedchangesintheproteinexpressionafterexposureto dibenzylbutyrolac-tolic(−)-6,6′-dinitrohinokinin.Weappliedtwo-dimensionalgelelectrophoresis(2-DE)toS.mansoni
solubleproteinextractsandobservedatleast38spotstobeaffectedbydibenzylbutyrolactolic(− )-6,6′-dinitrohinokinin.Wefurtheridentified25differentiallyexpressedproteinsbymassspectrometry.
Enrichmentforbiologicalprocessesandpredictiveanalysesofprotein-proteininteractionssuggestthat dibenzylbutyrolactolic(−)-6,6′-dinitrohinokinintargetsproteinsinvolvedmainlyinmetabolicprocesses,
especiallycarbohydratemetabolism.Insummary,thisstudyprovidesaninterestingapproachto under-standtheanti-parasiticactivityofsemi-synthetic(−)-6,6′-dinitrohinokininaderivativecompoundfrom
lignanandforthedevelopmentofnewtherapystrategies.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Schistosomiasisiscausedbyaparasiticinfectionwith trema-todeflukesof thegenusSchistosomaand affectsmorethan200 millionpeopleworldwide (Rollinsonet al.,2013).Currently, an antischistosomalvaccine is not available, whereas Praziquantel (PZQ)isthedrugofchoiceforthetreatmentofpatients(Caffrey, 2015).AlthoughPZQhavebeeneffectiveinthetreatmentofthe disease,thepossibilityofdrugresistancecausedbyrepeatedand massivemonotherapyreinforcestheneedtodevelopnewsafeand effectivedrugsforthepreventionandtreatmentagainst schistoso-miasis(Ciolietal.,2014;Colleyetal.,2014).
∗ Correspondingauthor.
E-mail:lizandra.magalhaes@unifran.edu.br(L.G.Magalhães).
Pipercubeba L.,Piperaceae, knownasthe tailed pepper, has
been used in traditional medicine for the treatment of dysen-tery,syphilis,abdominalpain,diarrhea,enteritisandasthma(Usia
etal.,2005;Gutierrezetal.,2013).Wedemonstratedpreviously
thatextractsandisolatedcompoundsfromPiperspeciespresent anti-parasitic activity (Saraiva et al., 2007, 2010; Esperandim et al., 2013). Furthermore,we showed that the essential oilof P. cubeba and their derivative dibenzylbutyrolactolic (−)-6,6′
-dinitrohinokinin(1,DNH),presentsinvitroandinvivoactivities againstSchistosomamansoni(Magalhãesetal.,2012;Pereiraetal.,
2015).
High-throughput strategies including genomics, transcrip-tomics, proteomics and metabolomics have become important approachestounderstandschistosomebiologyandpathogenesis, but also constituteimportant tools tothe exploration of novel drugs, vaccines or diagnosis (De Marco and Verjovski-Almeida,
2009; Chuan etal., 2010).In viewof thesefacts,we identified
http://dx.doi.org/10.1016/j.bjp.2016.02.001
changesintheproteinexpressioninducedbyexposuretoDNH. Weappliedastrategybasedontwo-dimensionalgel electrophore-sis(2-DE)followedbymassspectrometry,whichprovidedinsights intokeyproteins,molecularfunctionsandbiologicalprocesses tar-getedbyDNH.
Materialsandmethods
ObtainmentofDNH
Prior to the DNH synthesis, (−)-cubebin (2) was isolated fromPipercubebaL.,Piperaceae,seedsand(−)-hinokinin(3)was obtainedbyoxidationof(−)-cubebin(DaSilvaetal.,2005).(− )-Hinokinin was then submitted to a nitration reaction in the presenceoffumingHNO3.Thereactionwasprocessedunder
mag-neticstirringat0◦Cfor2h.TheobtainedDNH(1)wascrystallizedas
yellowishpowderfromMeOH.ThepurityofthesynthesizedDNH wasestimatedtobehigherthan95%bybothHPLCand1Hand13C
NMRanalyses,aswellasbyitsmeltingpoint(Setchelletal.,1981;
Costa,2000).
Yield 97.6%; [␣]D25 −29.07◦ (c 0.008, CHCl3, ee>99%); mp
191–193◦C;1HNMR(300MHz,CDCl
3)ı7.5(s,1H);ı7.48(s,1H);
ı6.8(s,1H);ı6.6(s,1H);ı6.1(m,2H);ı4.35(dd,1H,J=7.1Hz andJ=9.1Hz);ı4.0(dd,1H,J=7.3HzandJ=9.1Hz);ı3.26(d,2H, J=7.1Hz);ı3.2(dd,1H,J=6.3HzandJ=13.6Hz); ı3.0(dd,1H, J=7.8HzandJ=13.6Hz);ı2,8(m,2H);13CNMR(75MHz,CDCl
3):
178.0,152.3,152.2,147.6,143.1,142.9,130.9,130.7,112.5,111.2, 106.6,106.2,103.6,103.5,71.4,45.7,41.7,37.2,34.21.
Parasitestrain
TheLE(LuizEvangelista)strainofS.mansoniwasmaintained bypassagethroughBiomphalariaglabratasnailsandBALB/cmice. After49±2days,S.mansoniadultwormswererecoveredunder aseptic conditionsfrom micepreviouslyinfected with200 cer-cariaebyperfusionoftheirliversandmesentericveins(Smithers
andTerry,1965).ExperimentswereapprovedbyAnimalResearch
EthicsCommitteefromRibeirãoPretoMedicalSchool–University ofSãoPaulo,undertheprotocol021/2009.
S.mansonisolubleproteinextractionandtwo-dimensional polyacrylamidegelelectrophoresis(2-DE)
Parasitesexposedto25MDNHorRPMI1640medium(0.1% DMSO)weretransferredto0.5mlofhomogenizationbuffer[25mM Tris–HCl,pH7.5,1mMphenylmethanesulfonylfluorideand1mM dithiothreitol] and worms were lysed by sonication (5×10s, 21kHzat7mamplitude)at10sinterval,onice.Cellulardebris wasremovedfromthelysatebycentrifugationfor1hat15,000×g, 4◦C.Thesupernatantcontainingsolubleproteinswascollectedand
quantifiedusingtheCoomassieProteinAssayKit(Pierce Biotech-nology).
Solubleproteins(200g)weredissolvedinanisoelectric focus-ingbuffer[7Murea,2Mthiourea,4%(w/v)CHAPS,0.5%(v/v)carrier ampholytes(IPGbuffer3–10l,GEHealthcare),40mMdithiotreitol (DTT)and 0.002% (v/v)bromophenolblue] andrehydrated into
2
3
1
linear13-cmImmobilinedrystripspH3–10(GEHealthcare)for 16hat20◦C.IsoelectricfocusingwascarriedoutonEttanIPGphor
(GEHealthcare),byapplyingacurrentof50mA/strip,following the steps:1hat 500V (step), 1hat 1000V (gradient), 2.5hat 8000V(gradient)and0.5hat8000V(step),totaling16kVh.The focusedstripswereequilibratedfor15minin15mlequilibration buffer[50mMTris–HClpH8.8,6Murea,30% (v/v)glycerol,2% (w/v)SDS], supplementedwith1%(w/v) DTTandsubsequently for15minin15mlequilibration bufferwith2.5%(w/v) iodoac-etamide.Proteinswereseparatedin12.5%(w/v)sodiumdodecyl sulfatepolyacrylamidegelelectrophoresis(SDS-PAGE)usingthe Ruby600apparatus(GEHealthcare)andSDSelectrophoresisbuffer (25mMTris,192mMglycineand0.1%(w/v)SDS).Eachgelwas performedintriplicate.
Proteinvisualizationandimageanalysis
2-DEgelswerefixedfor1hwith3%(v/v)phosphoricacidand 50%(v/v)ethanol,followedbystainingwithCoomassieBrilliant BlueG250(GEHealthcare)during24h.2-DEproteinprofileswere obtainedaftergeldistaininginMilliQwater.Gelsweredigitized at300dpiand16bitsdepthresolutionwithanImageScanner(GE Healthcare)operatedbytheLabScansoftware(GEHealthcare).The softwareImageMaster2DPlatinum6.0(GEHealthcare)wasusedto mergetheimageofeachexperimentalreplicateontoamaster2-DE gelimage,usingthesixwell-definedlandmarkasreferencetospot detection.Differentialproteinexpressionwasanalyzedby compar-isonofspotintensityingelscontainingproteinsfromS.mansoni exposedtoDNHandunexposedcontrols.Spotsthatpresentedat least1.5-foldchangeinintensitygivenbyStudent’sttest(p<0.05) weremanuallyexcisedandanalyzedbymassspectrometry.
Ingeldigestionandidentificationofproteinsbyliquid
chromatography-tandemmassspectrometry(LC-MS/MS)
Spots obtained from 2-DE gels were digested with grade-modified trypsin (Promega), as described by Shevchenko et al.
(1996).Afterdigestion,peptideswereextractedwith50%(v/v)
ace-tonitrileplus5%(v/v)formicacidsolution.Theresultingpeptides weredriedagainandre-suspendedin12lof0.1%(v/v)formicacid. Analiquotof4.5lofthedigestedproteinwasthenseparatedona C18RP-UPLCcolumn(100mm×100mI.D.×1.7mparticlesize, nanoAcquityUPLC,Waters)usingalineargradientof15–90%(v/v) acetonitrilein0.1%(v/v)formicacidfor10minat0.6l/min.The HPLCsystemwascoupledtoaQ-TofUltimaAPImass spectrom-eter(MicroMass/Waters)fittedwithanano-electrospraysource. Theparameterswere35Vconevoltage,nano-electrospray volt-agewas3.5kV,and100◦Csourcetemperature.Theequipmentwas
250
A
3 pH 10B
3 pH 10Molecular mass (kDa)
150 100
75
50
37
25
20
15
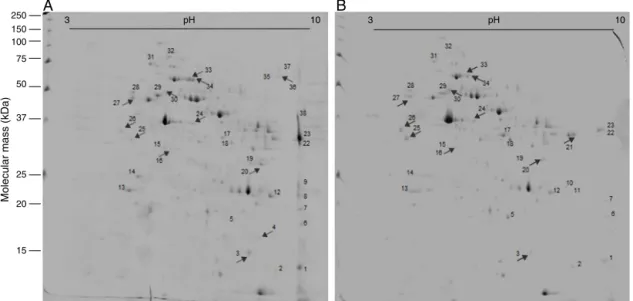
Fig.1.Effectof(−)-6,6′-dinitrohinokininonSchistosomamansoniadultproteinprofile.ComparativebidimensionalgelsanalysesofS.mansonisolubleproteinsbefore(A)
andafterexposureto25MDNH(B).Numbersrepresent38spotswhoseintensitywassignificantlyaffected(p<0.05)byDNH.
The data obtained were processed using Mascot Distiller v.2.3.2.0 (Matrix Science Ltd.) and proteins were identified by correlationoftandemmassspectraagainstpublicavailableS. man-soni(27,729 sequencesand12,093,356 residues) depositedinto NCBIdatabase.SearchparametersforMASCOTwereasfollows:S.
mansonitaxonomicrestriction;trypsincleavage,onemissed
cleav-age; carbamidomethyl–cys as fixedmodification (monoisotopic mass57.0215Da),methionineoxidationasvariablemodification (monoisotopicmass15.9949)carbamidomethyl(C);variable mod-ification,oxidation(M);peptidetolerance,0.1Da;MS/MSfragment iontolerance.Resultswereregardedassignificantwithanallowed likelihood for a random hit of p<0.05, according to the MAS-COT.
Bioinformaticanalysis
Identified proteins were analyzed for Gene Ontology (GO) enrichmentandproteinclassusingthebioinformaticstools PAN-THER (Mi et al., 2013) and UniProt (Consortium, 2014). The networksofinteractionfortheidentifiedproteinswereperformed using STRING database v 9.05, employing prediction meth-odsasneighborhood, genefusion,co-occurrence,co-expression, experiments, database and textmining sources (Jensen et al., 2009). We performed analysis in both Cluster of Orthologous Groups/EukaryoticOrthologousGroups(COG/KOG) (withahigh confidenceof0.700) andPROTEIN-modes(witha medium con-fidence of 0.400). The identity of the putative proteins were consideredsignificantiftheypresentedatleast75%ofhomology withsequencesfromSchistosomajaponicum.
Results
Differentialproteinanalysesrevealedapproximately38spots (i.e., proteins) that were significantly affected by 25M DNH (Fig.1Aand B).Most oftheseparated proteinshavemolecular weightsgreaterthan25kDaandaredistributedamongthe isoelec-tricpointsfrom4to10.Basicproteins(i.e.,proteinsthatpresent anisoelectricpointfrom8to10)weredirectlyaffectedby expo-suretoDNH,whichresultedintheirabsenceorlowerexpression. Although,weobservedthatspotsnumberedas4,8,9,19,35,36, 37and38arepresentinproteinprofileofS.mansoniunexposedto DNH,thesespotswerenotidentifiedafterdrugexposure(Fig.1A
andB).AfterexposuretoDNH,thespots2,5,10,11,17,18and21 werepresentorhadatleasta2-foldincreaseinexpression,while nineteenproteinshadatleasta2-foldreductionornoexpression (Fig.1AandB).
Togain furtherknowledge onchanges ofproteinexpression
byS.mansoniafterexposuretoDNH, weperformedLC-MS/MS.
Foreachspectrum,asearchforproteinsequencesfromS.mansoni wasperformedwithMASCOTatNCBInrproteindatabase(27,729 sequencesand12,093,356residues).Wefurtheridentified25 dif-ferentially expressed proteins by mass spectrometry, however, twentyfouridentifiedproteinshadMASCOTscoreshigherthan28 (p<0.05)andonlyoneproteinspot(spotn◦19)hadMASCOTscore
below28(p>0.05)(Table1).Ofinterest,thetegumentalantigenSm 20.8andphosphoglyceratemutase(spots10and11,respectively) wereexpressedfollowingexposuretoDNH,whereasthe peroxire-doxin,gelsolinandamajoreggantigen(spotsnumberedas5,17 and18,respectively)weretheproteinswiththehighest expres-sionafterexposuretoDNH(Fig.1AandB,andTable1).Notably, exposuretoDNHdecreasedtheexpressionofthepeptidyl-prolyl cis-transisomerase,glutathione-S-transferase(GST), 14-3-3 pro-tein,14-3-3epsilon,calreticulinandparamyosin(spotsnumbered as1,12,13,14,28and32,respectively)(Fig.1AandB,andTable1). MASCOTdidnotretrievesignificantmatchestospots3,4,16,20, 21,24,25,26,27,30,33,34and36,thereforewewereunableto identifytheirproteincontent(Fig.1).
Table1
SchistosomamansoniadultproteinsidentifiedbyLC-MS/MS.
Spotn◦ Proteinname Accessionnumbera MW(kDa)/pI MW(kDa)
experimental
Coverage%c MascotScored Expressionafter exposureDNHe
1 Peptidyl-prolylcis-trans
isomerase
Smp040130 18/8.26 12 46 390 Down
2 Nucleosidediphosphatekinase Smp092750 17/7.74 10 79 453 Up
5 Peroxiredoxin,Prx1 Smp059480 21/6.10 18 36 231 Up
6 Calponin-related Smp086330 21/8.68 17 55 453 Down
7 Putativeadenylatekinase Smp071390 22/8.52 19 45 331 Down
8 Triosephosphateisomerase Smp 003990 28/4.63 22 54 481 Down
9 Putativecarbonylreductase Smp033540 31/7.64 24 2 37 Down
10 Putativetegumentalantigen
Sm20.8
Smp086530 21/6.90 23 56 196 Up
11 Phosphoglyceratemutase Smp096760 28/7.71 22 50 234 Up
12 GlutathioneS-transferase Smp054160 24/6.56 22 16 132 Down
13 14–3-3protein,putative Smp 009760 28/4.74 23 46 553 Down
14 14–3-3epsilon Smp034840 28/4.85 25 52 354 Down
15 Camp-dependentprotein
kinasetypeII-alpharegulatory subunit
Smp079010 43/5.12 32 33 286 Down
17 Putativegelsolin Smp008660 42/5.57 34 46 433 Up
18 Putativemajoreggantigen Smp049300 39/6.23 33 18 287 Up
19 PutativefourandAhalflim domain
Smp048560 32/7.61 26 14 20 Down
22 Malatedehydrogenase Smp047370 37/8.70 34 31 746 Down
23 Glyceraldehyde-3-phosphate
dehydrogenase
Smp056970 37/8.16 36 32 305 Down
28 Calreticulinautoantigen
homolog,putative
Smp030370 45/4.69 49 39 231 Down
29 Putativealphatubulin Smp090120 51/4.97 49 53 1416 Down
31 Putativeheatshockprotein Smp072330 81/4.92 75 25 623 Down
32 Putativeparamyosin Smp 021920 90/5.3 98 14 142 Down
35 Taurocyaminekinase Smp194770 77/7.87 72 11 91 Down
37 Aconitatehydratase Smp063090 66/8.45 74 18 88 Down
38 Putativeuncharacterized
protein
Smp042170 40/7.63 38 20 167 Down
aS.mansoniGenedbdatabaseidentifier.
bPredictedmolecularweight(MW)andisoelectricpoint(pI)recoveredintheNCBIproteindatabaseandProtparamtool. c Coverage%:TheSequenceCoverageisthepercentageofthedatabaseproteinsequencecoveredbymatchingpeptides.
d Score:Relativefortheprobabilitythattheobservedmatchbetweentheexperimentaldataandthedatabasesequenceisarandomevent. eSpotsthatchangedatleast1.5-foldinintensity(downorup)afterexposuretoDNHcomparedtounexposedcontrol.
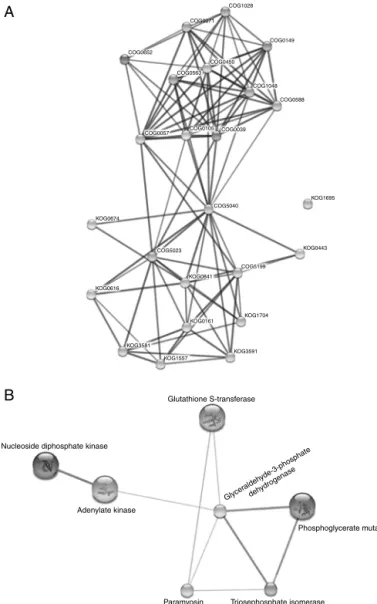
Next,tounderstandwhethertheidentified proteinsinteract witheachotherand thereforecontributetocommonbiological processes, we performed predictive protein-protein interaction analysesusingString.Analysisofdifferentiallyexpressedproteins
of S.mansoni assigned toclustersof orthologous groups (COG)
oreukaryoticorthologousgroups(KOG)showsthatallproteins, exceptforglutathione-S-transferase(GST)(KOG1695),might inter-actwithoneormorenodesinthepredictednetwork(Fig.3A).As expected,thenetworkdepictsanupperclusterofproteinsassigned withCOGs,whileinasecondcluster,themajorityofnodeswere assignedwithKOG.Furtheranalysisresultedina morespecific networkofpotentialtargetsinducedorrepressedbyexposureto DNH,whichpossesssignificantidentitywithproteinsofS. japon-icum(Fig.3B).Ofnote,fiveproteinsenrichedinthisnetworkwere reduced after exposureto DNH, while only nucleoside diphos-phatekinaseandphosphoglyceratemutasepresentedasignificant increaseinintensitywhencomparedtounexposedcontrols(Fig.1
andTable1).
Discussion
Here,weshowthatDNHmodulatestheprofileofaproteinset
fromS.mansoniadultworms.Severalofthoseproteins,affected
byexposuretoDNH,participateofimportantmetabolicprocesses andhavealreadybeenexploitedaspotentialvaccinecandidates
(Pearce,2003;Kohamaetal.,2010;Fonsecaetal.,2012;Beaumier
etal.,2013)orforimmunodiagnosis(ElAswadetal.,2011;Qian
etal.,2012;Yuetal.,2014).Forexample,reducedexpressionof
paramyosin,amajorstructuralcomponentthathasbeenassociated aspotentmucosalimmunogenagainstSchistosomassp.(Lanaretal.,
1986;Jizetal.,2009;Kohamaetal.,2010),mightreflectsimilar
activitywiththeessentialoilofP.cubebaandDNHbyaffecting themotilityofadultworms(Magalhãesetal.,2012;Pereiraetal.,
2015).
Analysisofprotein–proteininteractionsoperatedonCOG/KOG mode resulted in two major clusters of interactions, whereas proteins assigned to COG were mainly enriched in metabolic processes,suchasadenosine triphosphate(ATP)generationand carbohydrate metabolism(Fig.3A andTable 2).Forexample,S.
mansoninucleosidediphosphatekinase(COG0105)andadenylate
kinase(COG0563)catalyzereactionsthatgenerateATP(Senftand
Crabtree,1983).Ourresultsdemonstratedthatadenylatekinase
Table2
EnrichmentanalysisbyGeneOntologyandPANTHERterms.
IDsa Protein(COG/KOGIDs)b GOmolecularfunction GObiologicalprocess PANTHERproteinclass
Smp040130 Peptidyl-prolylcis-trans isomerase(COG0652)
Isomeraseactivity Proteinfolding,Intracellular
proteintransport,Nuclear transport
Isomerase
Smp092750 Nucleosidediphosphatekinase
(COG0105)
ATPbinding CTPbiosyntheticprocess,UTP
biosyntheticprocess
–
Smp059480 Peroxiredoxin,Prx1(COG0450) Oxidoreductaseactivity, Peroxidaseactivity
Metabolicprocess Peroxidase
Smp086330 Calponin-related(COG5199) – – –
Smp 071390 Putativeadenylatekinase
(COG0563)
Nucleotidekinaseactivity Purinenucleobasemetabolic process,Pyrimidinenucleobase metabolicprocess
Nucleotidekinase
Smp003990 Triosephosphateisomerase
(COG0149)
Isomeraseactivity Glycolysis Isomerase
Smp033540 Putativecarbonylreductase
(COG1028)
Oxidoreductaseactivity Steroidmetabolicprocess Dehydrogenase
reductase
Smp086530 Putativetegumentalprotein
Sm20.8
Catalyticactivity,Structural constituentofcytoskeleton, Proteinbinding,Enzyme inhibitoractivity
Nitricoxidebiosyntheticprocess, Nucleobase-containingcompound metabolicprocess,Cellcycle,RNA localization,Intracellularprotein transport,Vesicle-mediated transport,Regulationofcatalytic activity,Cellularcomponent organization
Enzymemodulator, Microtubulefamily, Cytoskeletalprotein
Smp096760 Phosphoglyceratemutase
(COG0588)
Bisphosphoglycerate 2-phosphataseactivity, Bisphosphoglyceratemutase activity,Phosphoglycerate mutaseactivity
Glycolyticprocess –
Smp054160 Glutathione-S-transferase (KOG1695)
Glutathionetransferase activity
Transferaseactivity –
Smp009760 14-3-3protein,putative
(COG5040)
Proteinbinding,ATPbinding, Proteindomainspecific binding
Cellcycle Chaperone
Smp034840 14-3-3epsilon(KOG0841) Proteinbinding,ATPbinding,
Proteindomainspecific binding
Cellcycle Chaperone
Smp 079010 Camp-dependentprotein
kinasetypeII-alpharegulatory subunit,putative(KOG0616)
Kinaseactivity,Protein binding,Kinaseregulator activity
Proteinphosphorylation Cellularprocess
Regulationofcatalyticactivity
Kinasemodulator
Smp008660 Putativegelsolin(KOG0443) Structuralconstituentof
cytoskeleton,Cytoskeletal proteinbinding
Cellularprocess,Cellular componentorganizationor biogenesis
Cytoskeletalprotein
Smp049300 Putativemajoreggantigen
(KOG3591)
– – –
Smp048560 PutativefourandAhalflim domains(KOG1704)
Zincionbinding,Metalion binding
– –
Smp047370 Malatedehydrogenase
(COG0039)
Oxidoreductaseactivity Generationofprecursor metabolitesandenergy, Carbohydratemetabolicprocess, Tricarboxylicacidcycle
Dehydrogenase
Smp056970 Glyceraldehyde-3-phosphate
dehydrogenase(COG00570)
Glyceraldehyde-3-phosphate dehydrogenase(NAD+) (phosphorylating)activity, NADandNADPbinding
Glucosemetabolicprocess Glycolysis
Oxidoreductase Dehydrogenase
Smp030370 Calreticulinautoantigen
homolog,putative(KOG0674)
Calciumionbinding Proteinfolding Calcium-binding
protein
Smp090120 Putativealphatubulin
(COG5023)
Structuralconstituentof cytoskeleton
Cellularcomponentmovement, Mitosis,Intracellularprotein transport,Cellularcomponent organization
Tubulin
Smp072330 Putativeheatshockprotein (COG0071)
ATPbinding Proteinfolding Chaperone
Smp021920 Putativeparamyosin
(KOG0161)
Motoractivity,Structural constituentofcytoskeleton, Proteinbinding,Enzyme regulatoractivity
Metabolicprocess,Cytokinesis, Cellularcomponentmovement, Mitosis,Cellcommunication, Musclecontraction,Sensory perceptionofsound,Sensory perception,Mesoderm development,Anatomical structuremorphogenesis, Intracellularproteintransport, Vesicle-mediatedtransport, Regulationofcatalyticactivity, Cellularcomponentorganization
Table2(Continued)
IDsa Protein(COG/KOGIDs)b GOmolecularfunction GObiologicalprocess PANTHERproteinclass
Smp194770 Taurocyaminekinase
(KOG3581)
ATPbinding,Metalionbinding, Oxidoreductaseactivity, Taurocyaminekinaseactivity
Catalyzes –
Smp063090 Aconitatehydratase
(COG1048)
Hydrolyaseactivity Generationofprecursor
metabolitesandenergy, Carbohydratemetabolicprocess, Tricarboxylicacidcycle,Cellular aminoacidbiosyntheticprocess
Dehydratase, Hydratase
Smp 042170 Putativeuncharacterized
protein(KOG1557)
– – –
aS.mansoniGenedbdatabaseidentifier.
bCOG,clusteroforthologousgroups;KOG,eukaryoticorthologousgroups;GO,GeneOntologyterms;PANTHERclassificationresultantfromenrichmentinUNIPROTand PATHERtools.
10
9
8
7
6
5
4
4 3
Proteins
Proteins
Category
Biological regulation (GO:0065007)
Cellular componente organization or biogenesis (GO:0071840) Cellular process (GO:0009987) Localization (GO:0051179) Metabolic process (GO:0008152)
Calcium-Binding protein (PC00060)
Antioxidant activity (GO:0016209) Binding (GO:005488)
Catalytic activity (GO:0003824) Enzyme regulator activity (GO:0030234) Structural molecule activity (GO:0005198) Kinase (PC00137)
Lyase (PC00144) Transferase (PC00220) Chaperone (PC00072) Cytoskeletal (PC00085) Enzyme modulator (PC00095) Isomerase (PC00135) Oxireductase (PC00176)
Category
Category
Proteins
3 2
2 1
1 0
0
10
9
8
7
6
5
4
3
2
1
0
A
B
C
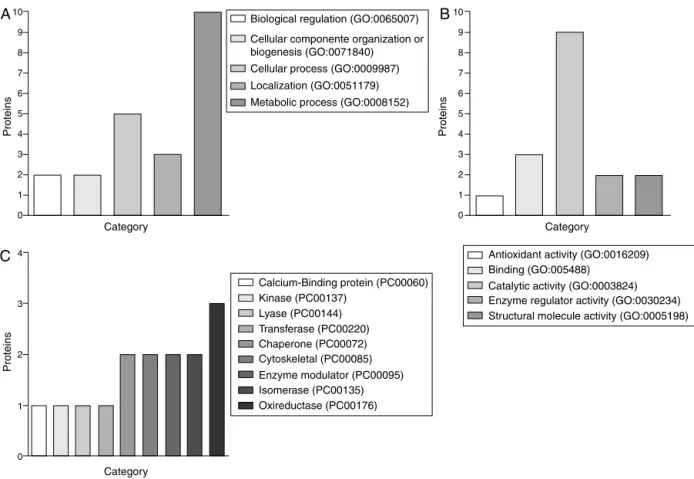
Fig.2.ClassificationofSchistosomamansoniadultproteinsaccordingtobiologicalprocessesandmolecularfunction.EnrichmentofGObiologicalprocesses(A),GOmolecular function(B)andPANTHERproteinclass(C)retrievedbyPANTHERclassificationsystem.
parasitichelminthesdependheavilyoncarbohydratemetabolism tosupplytheirenergydemand,whereasonequartertoonethirdof theirenergyderivesfromaerobicrespiration(Coles,1973;Barrett,
2009).
Evaluationof putativeinteractions onSTRING protein mode resultedinadistinctandsmallernetwork(Fig.3B).Paramyosin, GST,triosephosphateisomeraseandglyceraldehyde-3-phosphate dehydrogenaseandtheirreducedexpressionafterexposuretoDNH indicatesthatthoseproteinsparticipateofcommonbiological pro-cesses.Indeed,allofthemwereenrichedinmetabolicprocesses, whiletriosephosphateisomeraseandglyceraldehyde-3-phosphate dehydrogenasewerespecificallyassociatedtoglycolysis(Fig.3B
andTable2).PreviousstudydemonstratedthatS.mansoniGSThasa
lowlevelofhomologywithGSTfoundinmammalians,which rein-forcesitspotentialasatargetfordevelopmentofnewdrugsand vaccines(Tayloretal.,1988).Inaddition,severalanthelminticscan
alsobindtoglutathionetransferases,especiallythosecontaininga phenolicring(Barrett,2009).
Previousstudyrevealedthatperoxiredoxinissignificantlymore abundant after exposure to PZQ (Aragon et al., 2009), thus as expected,weobservedanincreaseinexpressionofthisenzyme afterexposuretoDNH.Overall,ourdatasuggestthatschistosomes exposedtoDNHmayincreasetheproductionofperoxiredoxinsasa defensemechanismagainstthestressandoxidativeprocess,which mightbeacommonprocessbetweenparasiticworms.
Acidicproteinsofthe14-3-3family arewidelyexpressedby
S.mansoni.Theseproteinsself-assemblespontaneouslyandform
phosphoserine–threonine-bindingdimersthat caninteractwith over 70 different proteins (Tzivion and Avruch, 2002). Studies haveshownthattheseproteinscouldbeinvolvedinthe regula-tionofPKCfunctionsduringthewormdevelopment(Wiestetal.,
A
B
Glutathione S-transferaseNucleoside diphosphate kinase
Adenylate kinase
Phosphoglycerate mutase
Triosephosphate isomerase Paramyosin
Glycer aldeh
yde-3 -phosphate
dehydrogenase
Fig.3. NetworkofinteractionsfromSchistosomamansoniproteinsaffectedby exposuretoDNH.IdentifiedS.mansoniproteinswerematchedtoS.japonicum
onSTRING databasev9.05.(A)NetworkofinteractionsonClusterof ortholo-gousgroups/Eukaryotic orthologousgroups (COG/KOG)mode. (B) Networkof interactionsonproteinmode;nucleosidediphosphatekinase,adenylatekinase, glu-tathioneS-transferase,glyceraldehyde-3-phosphatedehydrogenase,paramyosin, triosephosphateisomerase,phosphoglyceratemutase.
foundthat 14-3-3 epsilon interacts with the intra-cytoplasmic phosphorylated S.mansoni receptor kinase-1, but alsobinds to and modulatetheactivityof humantrans-forming growth fac-torreceptor-1(TRI)(McGonigleetal.,2001;Siles-Lucasand
Gottstein,2003).
Tegumentalantigensmediateseveralsignalingandtransport processes,suchastheregulationofcalciumlevelsandcontrolof musclecontraction(Mohamedetal.,1998).Therefore,these pro-teinsrepresentpotentialtargetstonewtherapycompoundsand vaccines(Fonsecaetal.,2012).DNHmayalsoactonparasite struc-ture,sinceweobservedahighexpressionofthetegumentalantigen Sm20.8inresponsetoDNH.Thishypothesisisfurthercorroborated bytheincreasedexpressionofgelsolinthathasbeenassociated withinhibitionofcellapoptosisbyblockingmitochondrial mem-branelossandpreventingthereleaseofcytochromeC(Koyaetal., 2000).Indeed,tegumentalantigenSm20.8,gelsolin,calponin,alpha tubulinand paramyosin areproteins particularlyimportant for theorganization ofthesyncytialtegumentofschistosomes and haveasignificantroleinhost-parasiteinteractions(Jonesetal.,
2004).
Overall,ourstudyaddsnewknowledgeintothe understand-ing of anti-schistosomicidal activity of semi-synthetic (−)-6,6′
-dinitrohinokininaderivativecompoundfromlignanandraisesnew perspectivesforfuturetherapeuticapproaches.
Authorcontributions
TCL, MLAS, RSL and JKB contributed by synthesis of DNH anddraftingthepaper.LGM,RGP,DPGandERMcontributedby proteomeanalysisanddraftingthepaper.LGM,LGGandGRG con-tributedbyanalyzingthedata.APY,VR,AASFandWRCsupervised theproteomeanalysisandcriticallyreadthemanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
The authors thank the Laboratório Nacional de Biociências, Brasil,CNPEM-ABTLUS,Campinas,Brazil,forsupportwiththemass spectrometricanalysis.ThisprojectwassupportedbyFundac¸ãode AmparoàPesquisadoEstadodeSãoPaulo,Brasil(grantnumbers 1998/14956-7and2010/17378-8andscholarships2009/15207-4, 2013/00382-0and2011/23819).
References
Aragon,A.D.,Imani,R.A.,Blackburn,V.R.,Cupit,P.M.,Melman,S.D.,Goronga,T., Webb,T.,Loker,E.S.,Cunningham,C.,2009.Towardsanunderstandingofthe mechanismofactionofpraziquantel.Mol.Biochem.Parasitol.164,57–65. Barrett,J.,2009.Fortyyearsofhelminthbiochemistry.Parasitology136,1633–1642. Beaumier,C.M.,Gillespie,P.M.,Hotez,P.J.,Bottazzi,M.E.,2013.Newvaccinesfor neglectedparasiticdiseasesanddengue.Transl.Res.J.Lab.Clin.Med.162, 144–155.
Caffrey,C.R.,2015.Schistosomiasisanditstreatment.FutureMed.Chem.7,675–676. Chuan,J.,Feng,Z.,Brindley,P.J.,McManus,D.P.,Han,Z.,Jianxin,P.,Hu,W.,2010.Our wormyworldgenomics,proteomicsandtranscriptomicsinEastandsoutheast Asia.Adv.Parasitol.73,327–371.
Cioli,D.,Pica-Mattoccia,L.,Basso,A.,Guidi,A.,2014.Schistosomiasiscontrol: praz-iquantelforever?Mol.Biochem.Parasitol.195,23–29.
Coles,G.C.,1973.Furtherstudiesonthecarbohydratemetabolismofimmature
Schistosomamansoni.Int.J.Parasitol.3,783–787.
Colley,D.G.,Bustinduy,A.L.,Secor,W.E.,King,C.H.,2014.Humanschistosomiasis. Lancet383,2253–2264.
Consortium,T.U.,2014.ActivitiesattheUniversalProteinResource(UniProt).Nucl. AcidsRes.42,D191–D198.
Costa,P.R.R.,2000.Safroleandeugenol:studyofthechemicalreactivityandusein thesynthesisofbiologicallyactivenaturalproductsanditsderivative.Quim. Nova23,357–369.
DaSilva,R.,deSouza,G.H.B.,DaSilva,A.A.,DeSouza,V.A.,Pereira,A.C.,Royo,V.D.A., ESilva,M.L.,Donate,P.M.,DeMatosAraújo,A.L.,Carvalho,J.C.,Bastos,J.K.,2005. Synthesisandbiologicalactivityevaluationoflignanlactonesderivedfrom(− )-cubebin.Bioorg.Med.Chem.Lett.15,1033–1037.
DeMarco,R.,Verjovski-Almeida,S.,2009.Schistosomes–proteomicsstudiesfor potentialnovelvaccinesanddrugtargets.DrugDiscov.Today14,472–478. ElAswad,B.E.D.W.,Doenhoff,M.J.,ElHadidi,A.S.,Schwaeble,W.J.,Lynch,N.J.,2011.
Useofrecombinantcalreticulinandcercarialtransformationfluid(CTF)inthe serodiagnosisofSchistosomamansoni.Immunobiology216,379–385. Esperandim,V.R.,DaSilvaFerreira,D.,SousaRezende,K.C.,Magalhães,L.G.,Medeiros
Souza,J.,Pauletti,P.M.,Januário,A.H.,daSilvadeLaurentz,R.,Bastos,J.K.,Símaro, G.V.,Cunha,W.R.,Andrade,E.,Silva,M.L.,2013.Invitroantiparasiticactivityand chemicalcompositionoftheessentialoilobtainedfromthefruitsofPipercubeba. PlantaMed.79,1653–1655.
Fonseca,C.T.,BrazFigueiredoCarvalho,G.,CarvalhoAlves,C.,deMelo,T.T.,2012. Schistosomategumentproteinsinvaccine anddiagnosisdevelopment:an update.J.Parasitol.Res.2012,541268.
Gutierrez,R.M.P.,Gonzalez,A.M.N.,Hoyo-Vadillo,C.,2013.Alkaloidsfrompiper: areviewofitsphytochemistryandpharmacology.MiniRev.Med.Chem.13, 163–193.
Jensen,L.J.,Kuhn,M.,Stark,M.,Chaffron,S.,Creevey,C.,Muller,J.,Doerks,T.,Julien, P.,Roth,A.,Simonovic,M.,Bork,P.,vonMering,C.,2009.STRING8–aglobal viewonproteinsandtheirfunctionalinteractionsin630organisms.Nucl.Acids Res.37,D412–D416.
(IgE)responsestoparamyosinpredictresistancetoreinfectionwithSchistosoma japonicumandareattenuatedbyIgG4.Infect.Immun.77,2051–2058. Jones,M.K.,Gobert,G.N., Zhang,L.,Sunderland, P.,McManus, D.P.,2004.The
cytoskeletonandmotorproteinsofhumanschistosomesandtheirrolesin sur-facemaintenanceandhost-parasiteinteractions.BioEssaysNewsRev.Mol.Cell. Dev.Biol.26,752–765.
Kohama,H.,Harakuni,T.,Kikuchi,M.,Nara,T.,Takemura,Y.,Miyata,T.,Sato,Y., Hirayama,K.,Arakawa,T.,2010.IntranasaladministrationofSchistosoma japon-icumparamyosininducedrobustlong-lastingsystemicandlocalantibodyas wellasdelayed-typehypersensitivityresponses,butfailedtoconferprotection inamouseinfectionmodel.Jpn.J.Infect.Dis.63,166–172.
Koya,R.C.,Fujita,H.,Shimizu,S.,Ohtsu,M.,Takimoto,M.,Tsujimoto,Y.,Kuzumaki,N., 2000.Gelsolininhibitsapoptosisbyblockingmitochondrialmembrane poten-tiallossandcytochromecrelease.J.Biol.Chem.275,15343–15349.
Lanar,D.E.,Pearce,E.J.,James,S.L.,Sher,A.,1986.Identificationofparamyosinas schistosomeantigenrecognizedbyintradermallyvaccinatedmice.Science234, 593–596.
Magalhães,L.G.,DeSouza,J.M.,Wakabayashi,K.A.L.,Laurentiz,R.,da,S.,Vinhólis, A.H.C.,Rezende,K.C.S.,Simaro,G.V.,Bastos,J.K.,Rodrigues,V.,Esperandim,V.R., Ferreira,D.S.,Crotti,A.E.M.,Cunha,W.R.,eSilva,M.L.A.,2012.Invitroefficacy oftheessentialoilofPipercubebaL.(Piperaceae)againstSchistosomamansoni. Parasitol.Res.110,1747–1754.
McGonigle,S.,Beall,M.J.,Feeney,E.L.,Pearce,E.J.,2001.Conservedrolefor 14-3-3epsilondownstreamoftypeITGFbetareceptors.FEBSLett.490,65–69. Mi,H.,Muruganujan,A.,Casagrande,J.T.,Thomas,P.D.,2013.Large-scalegene
function analysiswith the PANTHER classification system.Nat. Protoc.8, 1551–1566.
Mohamed,M.M.,Shalaby,K.A.,LoVerde,P.T.,Karim,A.M.,1998.Characterization ofSm20.8,amemberofafamilyofschistosometegumentalantigens.Mol. Biochem.Parasitol.96,15–25.
Pearce,E.J.,2003.Progresstowardsavaccineforschistosomiasis.ActaTrop.86, 309–313.
Pereira,A.C.,Silva,M.L.,Souza,J.M.,Laurentiz,R.S.,Rodrigues,V.,Januário,A.H., Pauletti,P.M.,Tavares,D.C.,Filho,A.A.,Cunha,W.R.,Bastos,J.K.,Magalhães, L.G.,2015.Invitroandinvivoanthelminticactivityof(−)-6,6′-dinitrohinokinin
againstschistosomulaandjuvenileandadultwormsofSchistosomamansoni. ActaTrop.149,195–201.
Qian,C.Y.,Wang,J.,Yu,C.X.,Yin,X.R.,Song,L.J.,Zhang,W.,Jin,Y.,Ke,X.D.,2012. Char-acterizationofIgGresponsesofrabbitstoSj14-3-3proteinafterexperimental infectionwithSchistosomajaponicum.Parasitol.Res.111,2209–2211.
Rollinson,D.,Knopp,S.,Levitz,S.,Stothard,J.R.,Tchuenté,L.A.,Garba,A.,Mohammed, K.A.,Schur,N.,Person,B.,Colley,D.G.,Utzinger,J.,2013.Timetosettheagenda forschistosomiasiselimination.ActaTrop.128,423–440.
Saraiva,J.,Veja,C.,Rolon,M.,daSilva,R.E.,Silva,M.L.A.,Donate,P.M.,Bastos,J.K., Gomez-Barrio,A.,deAlbuquerque,S.,2007.Invitroandinvivoactivityoflignan lactonesderivativesagainstTrypanosomacruzi.Parasitol.Res.100,791–795. Saraiva,J.,Lira,A.A.M.,Esperandim,V.R.,daSilvaFerreira,D.,Ferraudo,A.S.,
Bas-tos,J.K.,Silva,M.L.A.,deGaitani,C.M.,deAlbuquerque,S.,Marchetti,J.M.,2010. (−)-Hinokinin-loadedpoly(d,-lactide-co-glycolide)microparticlesforChagas disease.Parasitol.Res.106,703–708.
Senft,A.W.,Crabtree,G.W.,1983.Purinemetabolismintheschistosomes:potential targetsforchemotherapy.Pharmacol.Ther.20,341–356.
Setchell,K.D.R.,Borriello,S.P.,Gordon,H.,Lawson,A.M.,Harkness,R.,Morgan,D.M., Kirk,D.N.,Adlercreutz,H.,Axelson,M.,1981.Lignanformationinman–microbial involvementandpossiblerolesinrelationtocancer.Lancet2,4–7.
Shevchenko,A.,Wilm,M.,Vorm,O.,Mann,M.,1996.Massspectrometricsequencing ofproteinssilver-stainedpolyacrylamidegels.Anal.Chem.68,850–858. Siles-Lucas,M.D.M.,Gottstein,B.,2003.The14-3-3protein:akeymoleculein
para-sitesasinotherorganisms.TrendsParasitol.19,575–581.
Smithers,S.R.,Terry,R.J.,1965.Theinfectionoflaboratoryhostswithcercariae ofSchistosomamansoniandtherecoveryoftheadultworms.Parasitology55, 695–700.
Taylor,J.B.,Vidal,A.,Torpier,G.,Meyer,D.J.,Roitsch,C.,Balloul,J.M.,Southan,C., Sondermeyer,P.,Pemble,S.,Lecocq,J.P.,1988.Theglutathionetransferase activ-ityandtissuedistributionofaclonedMr28KprotectiveantigenofSchistosoma mansoni.EMBOJ.7,465–472.
Tzivion,G.,Avruch,J.,2002.14-3-3proteins:activecofactorsincellularregulation byserine/threoninephosphorylation.J.Biol.Chem.277,3061–3064. Usia,T.,Watabe,T.,Kadota,S.,Tezuka,Y.,2005.PotentCYP3A4inhibitory
con-stituentsofPipercubeba.J.Nat.Prod.68,64–68.
Wiest,P.M.,Burnham,D.C.,Olds,G.R.,Bowen,W.D.,1992.Developmentalexpression ofproteinkinaseCactivityinSchistosomamansoni.Am.J.Trop.Med.Hyg.46, 358–365.
Yu,Q.,Yang,H.,Guan,F.,Feng,Y.,Yang,X.,Zhu,Y.,2014.DetectionofIgGinseraof patientswithSchistosomiasisjaponicabydevelopingmagneticaffinity enzyme-linkedimmunoassaybasedonrecombinant14-3-3protein.Trans.R.Soc.Trop. Med.Hyg.108,37–41.