RevistaBrasileiradeFarmacognosia26(2016)281–284
w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Iridoid
and
phenylethanoid
glycosides
from
the
aerial
part
of
Barleria
lupulina
Seoung
Rak
Lee
a,
Jon
Clardy
b,
Donald
Robert
Senger
c,
Shugeng
Cao
d,∗,
Ki
Hyun
Kim
a,∗aSchoolofPharmacy,SungkyunkwanUniversity,Suwon,Gyeonggi-do,RepublicofKorea
bDepartmentofBiologicalChemistryandMolecularPharmacology,HarvardMedicalSchool,Boston,MA,USA
cDepartmentofPathologyandCenterforVascularBiologyResearch,BethIsraelDeaconessMedicalCenter,HarvardMedicalSchool,Boston,MA,USA
dDepartmentofPharmaceuticalSciences,DanielKInouyeCollegeofPharmacy,UniversityofHawaiiatHilo,Hilo,HI,USA
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received9December2015
Accepted11January2016
Availableonline1February2016
Keywords: Barlerialupulina
Acanthaceae
Iridoidglycoside
Phenolicglycoside
a
b
s
t
r
a
c
t
Anewiridoidglycoside,barlupulinCmethylester(1),togetherwithtwoknownphenylethanoid gly-cosides(2and3)andthreeknownsimplephenolicglycosides(4–6)wereisolatedfromtheaerialparts ofBarlerialupulinaLindl.,Acanthaceae.Thestructureofthenewcompound(1)waselucidatedthrough 1Dand2DNMRspectroscopicdata,andHR-ESIMS.Interestingly,compound(1)hasaformategroup attachedtotheC-6hydroxygroupoftheglucoseunit.Compounds2–6wereidentifiedaspoliumoside (2),decaffeoylacteoside(3),protocatechuicacid4-O--glucoside(4),vanillicacid4-O--glucoside(5), andleonurisideA(6)onthebasisofNMRspectroscopicdataanalysesandcomparisonwiththosereported intheliterature.Compounds3–6wereisolatedfromB.lupulinaforthefirsttime.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
The genusBarleria L.,a member of the Acanthaceae family,
isalargeandwidespreadgenusofherbsandshrubscomprising
approximately300species,growingmainlyinAfricaandAsia.The
plantsofthegenusBarleriahavebeenlongusedforboils,beebites,
andtooth-ache(AbdEl-Mawlaetal.,2005).BarlerialupulinaLindl.
isatinybushwidelydistributedanddomesticatedinthe
South-eastAsiaregion.InThaitraditionalmedicine,thisplanthaslong
beenusedasaprimaryanti-inflammatoryagentforinsectbites
andasaremedyforherpessimplexandvaricella zosterlesions
(Kanchanapoometal.,2001;Kimetal.,2015a).Previous
phyto-chemicalinvestigationsontheaerialpartsandleavesofB.lupulina
haveledtotheisolationofavarietyofcompoundsincluding
iri-doid glycosides, phenylpropanoid glycosides, lignan glucosides,
aliphaticglycosides,andbenzylalcohol glycosides(Byrne etal.,
1987;Tuntiwachwuttikuletal.,1998;Kanchanapoometal.,2001; Suksamrarnetal.,2003).
During our ongoing search for new bioactive metabolites
frommedicinalplants,recentlywereportedtheisolationoffour
newiridoidglycosides withfourteenknownanalogs and
4,8,8-trimethylcyclooct-2-enone derivatives with six known lignans
∗ Correspondingauthors.
E-mails:scao@hawaii.edu(S.Cao),khkim83@skku.edu(K.H.Kim).
fromthewaterextractsofB.lupulina(Kimetal.,2015a,b).Our
con-tinuedinterestindiscoveringnewcompoundsfromthisplantled
ustoisolateanewiridoidglycoside,barlupulinCmethylester(1),
togetherwithtwoknownphenylethanoidglycosides(2and3)and
threeknownsimplephenolicglycosides(4–6).Thestructureofthe
newcompound(1)waselucidatedthrough1Dand2DNMR
spec-troscopicdata,andHR-ESIMS.Tothebestofourknowledge,thisis
thefirstreportontheisolationofcompounds3–6fromB.lupulina.
Materialsandmethods
Generalexperimentalprocedures
OpticalrotationswereobtainedusingaJascoP-1010
polarime-ter. UV spectra were recorded on an Amersham Biosciences
Ultrospec5300Prospectrophotometer,andIRspectrawere
mea-sured ona BrukerAlpha-P spectrometer.AllNMR experiments
werecarriedoutonaVarianINOVA600NMRspectrometer.ESIMS
spectrawereobtainedbyLC/MSanalysiswhich wasperformed
onanAgilent1200SeriesHPLC/6130Seriesmassspectrometer.
HighresolutionmassspectrawereobtainedonaWaters
Micro-massQ-TofUltimaESI-TOFmassspectrometer.Allthecompounds
werepurifiedonanAgilent1100seriesHPLC(AgilentTechnologies)
usingaPhenomenexLunaphenyl-hexylcolumn(250mm×10mm,
5m particle size), a Phenomenex Luna phenyl-hexyl column
(250mm×21.2mm,10mparticlesize)andaPhenomenexLuna
http://dx.doi.org/10.1016/j.bjp.2016.01.002
282 S.R.Leeetal./RevistaBrasileiradeFarmacognosia26(2016)281–284
C18 column(250mm×21.2mm,5mparticlesize).Merck
pre-coatedsilicagelF254platesandRP-18F254splateswereusedforthin
layerchromatography(TLC).SpotsweredetectedonTLCunderUV
lightorbyheatingaftersprayingwithanisaldehyde–sulfuricacid.
Plantmaterial
TheaerialpartofBarlerialupulinaLindl.,Acanthaceae,was
pur-chasedatVungTauVietnam,inMarch,2012.Avoucherspecimen
(No.101)wasdepositedatBIDMC,HarvardMedicalSchool.
Extractionandisolation
Theair-driedaerialparts(200g)ofB.lupulinawereslicedand
boiledinwater(1.2l)for4–5hto100ml.Thissolutionwasthen
centrifugedat 10,000×g for 30minand filtered/sterilized. The
combinedextracts(200ml)weresuspendedinH2Oandthen
suc-cessivelypartitionedwithEtOAcandn-BuOH,yielding0.52gand
9gof residues,respectively. TheEtOAc-soluble fraction(0.52g)
wasfractionatedbypreparativeHPLC(C18column,Phenomenex
Luna,250mm×21.2mm,5m)using23%aqueousMeCN(0.1%
formic acid)for 20min,then to100% MeCN(0.1% formic acid)
in thenext 10min, and 100% MeCN(0.1% formic acid) for the
following 10min (flow rate: 10ml/min) to give eight fractions
(A–H)accordingtoHPLCchromatographyanalysis.FractionBwas
separatedbypreparativeHPLC(C18column,PhenomenexLuna)
using10%aqueousMeCNfor32min,thento100%MeCNinthe
next10min,and100%MeCNforthefollowing10min(flowrate:
10ml/min)toyieldtwelvefractions(B1–B12)accordingtoHPLC
chromatographyanalysis.FractionB4waspurifiedusinga
semi-preparative PhenomenexLuna phenyl-hexyl column (6%MeCN
with0.1% formicacid, flowrate:2ml/min)toyieldcompounds
4 (0.8mg, tR 29.6min) and 5 (0.6mg, tR 25.6min). FractionB6
wasseparatedusingasemi-preparativePhenomenexLuna
phenyl-hexylcolumn(7%MeCNwith0.1%formicacid,flowrate:2ml/min)
toaffordcompound6(0.8mg,tR19.7min).FractionB7was
sepa-ratedbysemi-preparativePhenomenexLunaphenyl-hexylcolumn
(10%MeCNwith0.1%formicacid,flowrate:2ml/min)toafford
compound3(0.9mg,tR15.0min).FractionB12wasseparatedby
semi-preparativePhenomenex Luna phenyl-hexyl column (13%
MeCNwith0.1%formicacid,flowrate:2ml/min)toafford
com-pound1(0.9mg,tR20.3min).FractionHwasfurtherseparatedby
preparativeHPLC(C18column,PhenomenexLuna)using40%
aque-ousMeCN(0.1%formicacid)for20min,thento60%MeCN(0.1%
formicacid)inthenext10min,and100%MeCN(0.1%formicacid)
forthefollowing10min(flowrate:10ml/min)toyield39
frac-tions(H1–H39)accordingtoHPLCchromatographyanalysis.The
combinedmixtureoffractionsfromH5toH9(assignedasK)was
furtherseparatedusingapreparativePhenomenexLuna
phenyl-hexylcolumn(250mm×21.2mm,10mparticlesize)using10%
aqueousMeCN(0.1%formicacid)for30min,thento100%MeCN
(0.1%formicacid)inthenext5min,and100%MeCN(0.1%formic
acid)forthefollowing10min(flowrate:10ml/min)toyield39
subfractions(K1–K39).Theconsolidatedmixtureoffractionsfrom
K36toK38wasseparatedusingapreparativePhenomenexLuna
phenyl-hexylcolumn(21%MeCNwith0.1%formicacid,flowrate:
10ml/min)togivecompound2(1.8mg,tR13.5min).
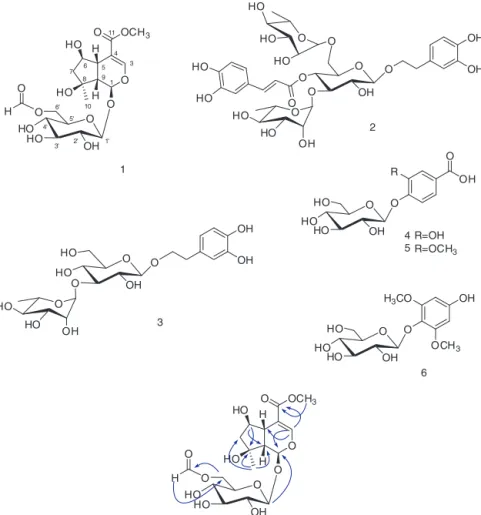
BarlupulinCmethylester(1)
Amorphouspowder.[␣]D25−35.8(c0.05,MeOH);IR(KBr)max
3375,2924,1657,1597,1452,1352,1276,1170,1025cm−1;UV
(MeOH)max(logε)236(3.56)nm;1H(CD3OD,600MHz)and13C
NMR(CD3OD,150MHz)data,seeTable1;positiveHR-ESIMSm/z
457.1317[M+Na]+(calcd.forC
18H26O12Na,457.1322).
Acidhydrolysisof1
Compound1(0.5mg)wasrefluxedin6%HCl(1ml)at80◦Cfor
2h.ThereactionmixturewasextractedwithCHCl3(3×6ml),and
theH2Ophasewasdriedusingaspeedvacconcentrator.Thedried
water-solubleresiduewasseparatelysubjectedtocolumn
chro-matographyoversilicagelwithEtOAc–EtOH–H2O(7:4:1)asan
eluent,toyieldglucose(0.1mg),whichshowedtheoptical
rota-tion,[␣]D25+42.5(c0.01,H2O).TLCidentificationofglucosewas
analyzedbysilicagelco-TLCwithanauthentic sample[solvent
system(CHCl3–MeOH–H2O,8:5:1),Rfofglucose,0.30](Kimetal.,
2011).
Resultsanddiscussion
Thepresentstudyreportstheisolationandidentificationofan
iridoidglycoside(1),twophenylethanoidglycosides(2and3),and
threesimplephenolicglycosides(4–6)fromtheaerialpartsofB.
lupulina.Theiridoidglycoside(1)wascharacterizedasanew
com-pound.Compounds4–6wereisolatedfromthegenusBarleriafor
thefirsttime,andcompound3wasisolatedfromB.lupulinaforthe
firsttime.
Compound 1 wasisolated as an amorphous powder,[␣]D25
−35.8(c0.05,MeOH).Themolecularformulawasdeterminedto
beC18H26O12,bythemolecularionpeakatm/z457.1317[M+Na]+
(calcd.forC18H26O12Na,457.1322)inthepositive-ionHR-ESIMS
and 13C NMR data. TheIR spectrum displayed thepresence of
hydroxy(3375cm−1)andcarbonyl(1657cm−1)groupsandanenol
ethersystem(1597cm−1).The1HNMRspectrumof1(Table1)
showedsignalsforonemethylgroupatıH1.24(3H,s),onemethoxy
groupatıH3.72(3H,s),oneanomericprotonatıH4.65(1H,d,
J=8.5Hz),one olefinicproton atıH 7.39(1H,s), andone
alde-hydeprotonatıH 8.14(1H,s).The13C NMRandHSQC spectra
for1showed18carbonsignalsclassifiedastwomethyls
(includ-ingonemethoxygroup),onemethylene,fivemethines(including
threeoxygenated),threequaternarycarbons(includingone
oxy-genated),onealdehydegroup,andsixcarbonsignals(includingone
oxygenatedmethyleneandfiveoxygenatedmethines),indicating
ahexoseresidue.
ThecomparisonoftheNMRdataof1withthosereportedfor
iridoidglycosidesrevealedthatcompound1hasasimilarstructure
Table1
1H(600MHz)and13CNMR(150MHz)dataofcompound1inCD3OD.a
Position 1
ıC ıH(JinHz)
1 95.4d 5.40,d(3.5)
3 153.0d 7.39,s
4 111.5s
5 42.3d 3.01,dd(10.0,4.0)
6 78.1d 4.03,m
7␣ 49.5t 2.00,dd(13.0,6.0)
7ˇ 1.82,dd(14.0,6.0)
8 79.4s
9 52.1d 2.57,dd(10.0,3.5)
10 24.8q 1.24,s
11 169.4s
OCH3 52.2q 3.72,s
1′ 100.6d 4.65,d(8.5)
2′ 74.9d 3.17,m
3′ 78.0d 3.34,m
4′ 71.8d 3.30,m
5′ 75.8d 3.50,m
6′a 64.2t 4.51,dd(12.0,2.0)
6′b 4.28,dd(12.0,6.0)
6′-COH 163.4s 8.14,s
aTheassignmentswerebasedon1H–1HCOSY,HSQC,TOCSY,andHMBC
S.R.Leeetal./RevistaBrasileiradeFarmacognosia26(2016)281–284 283
O O OCH3
O H
H HO
HO
O HO
HO
OH O
H
O 1
5 3
4
6 7
8 9
10 11
1' 2' 3' 4'
5' 6'
1
OH
OH O
O O
O OH
O
O HO
HO
O
OH HO HO
O
HO HO HO
2
OH
OH O
O HO
O OH
O
OH HO HO
HO
3
R
O O
HO
HO OH
HO
O H O
H3CO
O O
HO
HO OH
HO
O CH3 OH
6 4R=OH 5 R=OCH3
O O OCH3
O H
H HO
HO
O HO
HO
OH O
H O
Fig.1.KeyHMBCcorrelationsofcompound1.
tobarlupulinCisolatedfromthisplant,withtheexceptionofthe appearanceofa methoxygroup(Jensenetal.,2007;Kim etal., 2015a).ThepositionofthemethoxygroupwasassignedtoC-11by
theHMBCcorrelationsbetweenıH3.72andıC169.4(C-11)(Fig.1).
Meanwhile,thepositionoftheestergroup(C-11)wasconfirmed
byHMBCcorrelationsfromıH7.39(H-3)andıH3.01(H-5)toıC
169.4(C-11).Acidhydrolysisof1affordedd-glucose,whichwas
identifiedbyTLCcomparisonwithanauthenticsample(Kimetal.,
2015a),andtheconfigurationwasdeterminedbycomparisonof
opticalrotationdata.The-anomericconfigurationfortheglucose
wasdeterminedbythecouplingconstantofanomericproton(d,
J=8.5Hz).Thelocationofthed-glucosewasdeterminedonthe
basis of HMBC correlation betweenıH 4.65(H-1′)and ıC 95.4
(C-1).Therelativeconfigurationof1wasconfirmedbyanalysis
oftheNOESY spectrumwhereNOESYcorrelationsbetweenH-9
andH-5/H-7ˇindicatedthatH-5andH-9arebothˇ-oriented,and
NOESYcorrelationsbetweenH-10andH-1/H-6/H-7˛impliedthat
H-1,H-6, andH-10areall˛-oriented.The1H–1HCOSY,TOCSY,
HMBC,andNOESYspectraanalysis(Fig.1)allowedustoestablish
the complete structure of 1, as shown in Fig. 1. Interestingly,
compound 1 hasa formate groupattached totheC-6 hydroxy
groupoftheglucoseunit.Iridoidglycosideswithaformategroup
haveyettobereportedinotherhigherplants,howevertheywere
recentlyisolatedfromB.lupulina(Kimetal.,2015a).Thisfinding
suggeststhattheoccurrenceofiridoidglycosideswiththeformate
groupcanserveasachemotaxonomicmarkerforB.lupulina.
Compounds2–6wereidentifiedaspoliumoside(2)(Akdemir
etal.,2004),decaffeoylacteoside(3)(Kimetal.,2009),
protoca-techuicacid4-O--glucoside(4)(Singabetal.,2011),vanillicacid
4-O--glucoside(5)(Cuietal.,1993),andleonurisideA(6)(Otsuka
etal.,1989),respectively,onthebasisofNMRspectroscopicdata
analysesandcomparisonwiththosereportedintheliterature.
Conclusions
ThephytochemicalinvestigationoftheaerialpartsofB.lupulina
afforded anewiridoid glycoside,barlupulin Cmethylester (1),
togetherwithtwoknownphenylethanoid glycosides(2 and3);
poliumoside(2)anddecaffeoylacteoside(3),andthreeknown
sim-plephenolicglycosides(4–6);protocatechuicacid4-O--glucoside
(4),vanillicacid4-O--glucoside(5),andleonurisideA(6).
Com-pound1hasaformategroupattachedtotheC-6hydroxygroup
oftheglucoseunit,whichsuggestedthatthestructuralfeatureof
theformategroupiniridoidglycosidesmayserveasanimportant
chemotaxonomicmarkerofB.lupulina.Compound3wasisolated
fromB.lupulinaforthefirsttime,andcompounds4–6wereisolated
fromthegenusBarleriaforthefirsttime.
Authors’contribution
SRLcontributedtotheexperimentandwrotethemanuscript.JC
reviewedthemanuscript.DRSconductedtheexperiment.SCand
KHKcontributedtothedesignofthestudyandcriticalreadingof
themanuscript.Alltheauthorshavereadthefinalmanuscriptand
approvedthesubmission.
Conflictsofinterest
284 S.R.Leeetal./RevistaBrasileiradeFarmacognosia26(2016)281–284
Acknowledgements
This publication was made possible by grant number
R01AT007022(toD.S.andS.C.)fromNationalCenterfor
Comple-mentaryandIntegrativeHealth(NCCIH),thentheNationalCenter
for Complementary and Alternative Medicine (NCCAM), at the
NationalInstitutesofHealth,USA.Thisresearchwasalsosupported
byBasicScienceResearchProgramthroughtheNationalResearch
FoundationofKorea(NRF)fundedbytheMinistryofScience,ICT&
FuturePlanning(2015R1C1A1A02037383).
References
AbdEl-Mawla,A.M.A.,Ahmed,A.S.,Ibraheim,Z.Z.,Ernst,L.,2005.Phenylethanoid
glycosidesfromBarleriacristataL.calluscultures.Bull.Pharm.Sci.Assiut Uni-versity28,199–204.
Akdemir,Z.S.,Tatli,I.I.,Bedir,E.,Khan,I.A.,2004.Iridoidandphenylethanoid
glyco-sidesfromVerbascumlasianthum.Turk.J.Chem.28,227–234.
Byrne,L.T.,Sasse,J.M.,Skelton,B.W.,Suksamrarn,A.,White,A.H.,1987.Theminor
iridoidglucosidesofBarlerialupulina:isolation,crystalstructureandplant growth-inhibitingpropertiesof6-O-acetylshanzhisidemethylester.Aust.J. Chem.40,785–794.
Cui,C.B.,Tezuka,Y.,Yamashita, H.,Kikuchi,T., Nakano,H.,Tamaoki,T.,Park,
J.H.,1993.Constituentsofafern,DavalliamariesiiMoore.V.Isolationand
structures of davallin, a new tetrameric proanthocyanidin, and twonew phenolicglycosides.Chem.Pharm.Bull.41,1491–1497.
Jensen,S.R.,Calis,I.,Gotfredsen,C.H.,Sotofte,I.,2007.Structuralrevisionofsome
recentlypublishediridoidglucosides.J.Nat.Prod.70,29–32.
Kanchanapoom,T.,Kasai,R.,Yamasaki,K.,2001.IridoidglucosidesfromBarleria
lupulina.Phytochemistry58,337–341.
Kim,K.H.,Kim,S.,Jung,M.Y.,Ham,I.H.,Whang,W.K.,2009.Anti-inflammatory
phenylpropanoid glycosides from Clerodendron trichotomum leaves. Arch. Pharm.Res.32,7–13.
Kim,K.H.,Kim,H.K.,Choi,S.U.,Moon,E.,Kim,S.Y.,Lee,K.R.,2011.Bioactivelignans
fromtherhizomesofAcorusgramineus.J.Nat.Prod.74,2187–2192.
Kim,K.H.,Park,Y.J.,Chung,K.H.,RichardYip,M.L.,Clardy,J.,Senger,D.,Cao,S.,2015a.
IridoidglycosidesfromBarlerialupulina.J.Nat.Prod.78,320–324.
Kim,K.H.,Clardy,J.,Senger,D.,Cao,S.,2015b.ChakyunglupulinsAandBtwonovel
4,8,8-trimethylcyclooct-2-enonederivativesfromBarlerialupulina.Tetrahedron Lett.56,2732–2734.
Otsuka, H., Takeuchi, M., Inoshiri, S., Sato, T., Yamasaki, K., 1989.
Pheno-liccompounds from Coixlachryma-jobivar. Ma-yuen.Phytochemistry 28, 883–886.
Singab,A.N.B.,El-Ahmady,S.H.,Labib,R.M.,Fekry,S.S.,2011.Phenolicsfrom
Kalan-choemarmorataBaker.FamilyCrassulaceae.Bull.Fac.Pharm.CairoUniv.49, 1–5.
Suksamrarn, S., Wongkrajang, K., Kirtikara, K., Suksamrarn, A., 2003.
Iri-doid glucosides from the flowers of Barleria lupulina. Planta Med. 69, 877–879.
Tuntiwachwuttikul,P.,Pancharoen,O.,Taylor,W.C.,1998.Iridoidglucosidesof