w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Effects
of
Acmella
oleracea
methanolic
extract
and
fractions
on
the
tyrosinase
enzyme
Alan
F.
Barbosa
a,∗,
Keila
C.B.
Silva
b,
Márcia
C.C.
de
Oliveira
b,
Mário
G.
de
Carvalho
b,
Armando
U.O.
Sabaa
Srur
a,caDepartmentofFoodTechnology,InstitutodeTecnologia,UniversidadeFederalRuraldoRiodeJaneiro,Seropédica,RJ,Brazil
bDepartmentofChemistry,InstitutodeCiênciasExatas,UniversidadeFederalRuraldoRiodeJaneiro,Seropédica,RJ,Brazil
cDepartmentofBasicandExperimentalNutrition,InstitutodeNutric¸ãoJosuédeCastro,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received23July2015
Accepted20January2016
Availableonline23February2016
Keywords: Jambu
Acmellaoleracea
Spilanthol Tyrosinase
a
b
s
t
r
a
c
t
TheaimofthecurrentstudyistoevaluatetheeffectofAcmellaoleracea(L.)R.K.Jansen,Asteraceae,
methanolicextract,hexane(84.28%spilanthol)anddichloromethane(approximately100%spilanthol)
fractionsonthetyrosinaseenzyme.Thedehydratedjambuextractwasobtainedthroughmaceration
usingmethanol.TheextractresiduewassolubilizedinMeOH/H2O(8:2)andsubjectedtoliq.–liq.partition inorganicsolvents.Boththeextractionandthepartitionprocedureswereconductedwiththree repli-cates.TheanalyseswereperformedusingGC–MS,1Hand13CNMR.Thehexanefractionprovidedsamples containing84.28,82.91and62.83%spilantholinrepetitions1,2and3,respectively.Thedichloromethane fractionshowed88.55%spilantholinrepetition1,andapproximately100%spilantholinrepetitions2and 3.Thejambuextractaswellasthehexanefraction(84.28%spilanthol)wereabletoactivatethe
oxidiz-ingactivityofthetyrosinaseenzymeforl-DOPA.Thedichloromethanefraction(approximately100%
spilanthol)showedstrongerinhibitioneffectonthetyrosinaseenzymeinthefirst10min.Theresults raisetheinterestinstudyinspilantholformulationsfortopicaluse,sinceitmaypreventand/orslow
skinhyperpigmentationordepigmentationprocesses.Furthermore,spilantholmaybeusedtocontrol
theenzymaticbrowninginfruitsandvegetables.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Acmellaoleracea(L.)R.K.Jansen,Asteraceae,isanative
Amazo-nianplantpopularlyknownasjambu.Itisoftenusedascondiment
intypicaldishesoftheNorthernBraziliancuisine,suchastacacá
andpato-no-tucupi (duck intucupisauce).It is alsousedinfolk
medicinetotreatstomatitis,coldsandgeneralpain(Nascimento
etal.,2013).A.oleraceaisconsideredtobeanimportantnative
plant in the Amazon region, and it is found in large
cultiva-tionareas(Rebelloand Homma,2005).Spilanthol hasbeenthe
mainmetaboliteoftenisolatedfromA.oleracea.Itisanaliphatic
amidedescribed asaburningviscousoil,which produces
anes-thetic effect and tongue tingling (Molina-Torres et al., 1996),
anditisalsoabletopenetratetheskin(Boonenetal.,2010a,b;
Spiegeleer et al., 2013).Besides Spilanthol’s anti-wrinkleeffect (Demarneand Passaro, 2005), it is alsopossiblementioningits
∗ Correspondingauthor.
E-mail:alanfbarbosa@ufrrj.br(A.F.Barbosa).
diuretic(Ratnasooriyaetal.,2004),fungistaticandbacteriostatic
activities(Molina-Torresetal.,2004),sensoryproperties(Leyetal.,
2006),antisepticactivity,immunestimulation(Rojasetal.,2006),
antioxidantandanti-inflammatoryproperties(Diasetal.,2012),
saliva-secretioninduction(Ramsewaketal.,1999;Sharmaetal.,
2011),analgesic(Riosetal.,2007),andacaricideactivity(Castro
etal.,2014),aswellitsuseagainstskindiseasessuchaseczema
(Boonenetal.,2010a,b).
A.oleraceahasimportantchemicalpropertiesthatawakened
theinterestofthepharmaceuticalindustryduetoitsactive
ingre-dient, spilanthol (Borges et al., 2012). Currently the search for
naturalproductswithinhibitoryactiononmelanizationprocess
hasincreased,focusingonthephenoloxidasetyrosinase.
Tyrosinase,alsoknownaspolyphenoloxidase(PPO),iswidely
distributedinmicroorganisms,animalsandplants.Itcatalyzesthe
oxidation of monophenols, o-diphenolsand o-quinones(Karioti
et al.,2007).Tyrosinase is knownas a key enzyme inmelanin
biosynthesis and it is responsible for melanization in animals
and for browningin plants.Tyrosinase is responsiblefor
enzy-maticbrowningreactionsindamagedfruitsduringpost-harvest
http://dx.doi.org/10.1016/j.bjp.2016.01.004
handlingandprocessing.Thus,controllingtheenzymatic
brown-ingisessentialduringfruitpulpmanufacturingprocesses(Seoetal.,
2003;Khanetal.,2006).
Tyrosinasesynthesisoccursinsidehighlyspecializedorganelles
calledmelanosomes.Studieshaveshownthepresenceof
tyrosi-nase in all the evaluated melanomas; fact that proves this
enzyme’s importance to the development of this cancer type
(Figueiredo,2003).Theincreasedproductionandaccumulationof
melaninhyperpigmentationmayleadtodisorderssuchasmelasma
(Miotetal.,2009).Manychemicalsandfoodhavedemonstrated
inhibitoryeffectonmelanogenesisthroughtheinhibitionofthe
tyrosinaseenzymeactivity.Suchresulthasincreasedthedemand
fornaturalproducts.Thus,theaimofthecurrentstudyisto
eval-uatethe effectof A.oleraceamethanol extract, hexane (84.28%
spilanthol)anddichloromethane(approximately100%spilanthol)
fractionsonthetyrosinaseenzyme.
Materialsandmethods
Jambusamples
Theplantmaterial(leaves,stemsandinflorescences)fromthe
jambu samples was collected in Igarapé-ac¸u County, which is
locatedinBragantinaRegion,intheNortheasternParáState,Brazil,
atthecoordinates:01◦07′33′′Sand47◦37′27′′W(Oliveiraetal.,
2011).Theplant(MG205534)wasidentifiedasAcmellaoleracea(L.)
R.K.Jansen,Asteraceae,anditwasincorporatedtotheherbarium
ofEmílioGoeldiMuseum,Belém,ParáState.
Theplantwasinitiallywashedinwatertoremovesoilresidues,
therootswereremovedusingstainlessknives,andtheplant’storn
andcrumpledparts,aswellasthosewithdarkenededgeswere
eliminatedfromthedryingprocess.Therawmaterialswere
sani-tizedthroughimmersioninsolutioncontaining200ppm(mgl−1)
offreeresidualchlorine(FRC)derivedfromsodiumhypochlorite
with10%purity,for10min.Thelastrinsewasperformedthrough
immersioninsolutioncontaining5ppm(mgl−1)FRC,for10min,
andsubsequentwaterdrainage.
Thecold-dryingprocesswascarriedoutinacclimatizedroom
usingairconditioning(Midea,modelMS2E-18CR,Brazil)at25◦C,
anddehumidifier(Arsec,model160,Brazil).Theroommeasured
4m2andremainedclosedduringthedryingprocedure.
Extractionprocedure
ThedriedA.oleraceaplantmaterialwascrushedandsubjected
toan exhaustive extraction process through methanol(MeOH)
macerationatroomtemperature(Mbeunkuietal.,2011).The
sol-vent was removedin rotary evaporator at 40◦C under reduced
pressure.Themethanolusedshowed99.8%purityandextraction
wasperformedforabout30dayswithapproximately6lofsolvent.
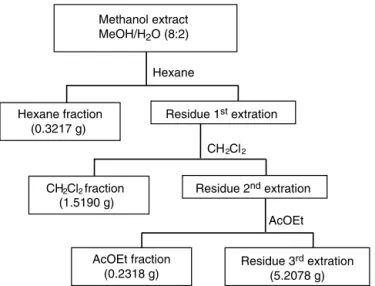
TheMeOHextractwassolubilizedinMeOH/H2O(8:2)andthe
solutionwassubjectedtosuccessiveextractionsinseparatory
fun-nelwiththesolvents:n-hexane,dichloromethane (CH2Cl2)and
ethylacetate(AcOEt),asshowninFig.1.Threereplicationswere
performedtoobtaintheextractandtheliq.–liq.extraction.
Tyrosinaseenzymeactivity
The reagents employed in the inhibitory investigationwere
obtainedatSigma–Aldrich.
TyrosinaseinhibitionactivitywasmeasuredbyamodifiedPatil
andZucker(1965)UV–vismethod.Themodificationsconsistedon
theuseofa differentconcentrationofEDTA. Also,l-DOPAwas
employedasa substrateandfinally,commercialtyrosinasewas
usedwhereastheauthorsisolatedtheenzyme.
Hexane fraction (0.3217 g)
Residue 1st extration
Residue 2nd extration CH2Cl2fraction
(1.5190 g)
AcOEt fraction (0.2318 g)
Residue 3rd extration (5.2078 g) Methanol extract
MeOH/H2O (8:2)
CH2Cl2
AcOEt Hexane
Fig.1. FractionationoftheAcmellaoleraceamethanolextractinthesecond
repeti-tion.
Methanolicextractfromjambu,hexanefraction(84.28%
spilan-thol)anddichloromethane(containingapproximately100%
spilan-thol)fractionweresolubilizedindimethylsulfoxide(10mgml−1)
anddifferentaliquotsofthesolutionwereaddedtothereaction
mediumcontainingthetyrosinaseenzyme(50–100units),EDTA
(0.022mmoll−1),l-DOPA(0.17mmoll−1)inPBS(50mmoll−1,pH
8.0) in room temperature. The time for this phenolase to
oxi-dizel-DOPA was30min afterthe reaction time readingswere
performedinaspectrophotometer(Shimadzu,modelMini1240,
Japan)UV–visat475nm.
The otherexperiment evaluated the action of themethanol
extractedfromjambubyusingtheenzymereactionmediumwith
directincidenceofultravioletirradiationat312nm(usedUVlamp)
at10minintervalsfor30min.
Theconcentrations0.66and0.16mgml−1(methanolicextract);
0.51–0.05mM(hexanefractioncontainingapproximately84.28%
spilanthol) and 0.53mM (dichloromethane fraction containing
approximately100%spilanthol)usedintheenzymeactivity
eval-uationtest wereusedtodeterminetheenzyme kineticsinthe
presenceofsample.Theevolutionofthereactionwasmonitoredby
readingstakeninUV–visspectrophotometerat475nmfor30min
and60minin10minintervals.
Theactivationandinhibitionvalueswerecalculatedfromthe
belowequation:
%inib=
[(B30−B0)−(A30−A0)](B30−B0)
×100 (1)
where B0=absorbance of l-DOPA+tyrosinase at t=0min,
B30=absorbance of l-DOPA+tyrosinase at time=30min, A0=
absorbance of l-DOPA+tyrosinase+inhibitor/activator at time=
0min, and A30=absorbance of l-DOPA+tyrosinase+inhibitor/
activatorattime=30min.
Theaboveequationallowstheevaluationoftheactionofplant
extractsandorganiccompoundsontheenzymetyrosinase,aswhat
int=0min.Thepossibleabsorptionofthetestsamplesat475nm
(whicharerelatedtoproductiondopacromona)issubtracted.
Allexperimentswereperformedintriplicate,andtheresults
wereexpressedasmeans±SD.Thegraphicswasfitofthe
experi-mentaldatainOriginsoftware(ANOVAstatisticalfunction).
Chemicalanalysis
The material was analyzed through a gas chromatograph
coupledtoamassspectrometer–GC/MS(Shimadzu,model
3.0
a
b
2.01.0
100 90 80 70 60 50
30 40 41
53 69 81
85 98
115 126
141
167 178 192 206 221
50 60 70 80 90 100 110 120 130
m/s 140 150 160 170 180 190 200 210 220 40
30 20 10
2.5 5.0 7.5 10.0 12.5 Spilanthol TIC
(x1 000 000)
15.0 17.5 20.0 22.5
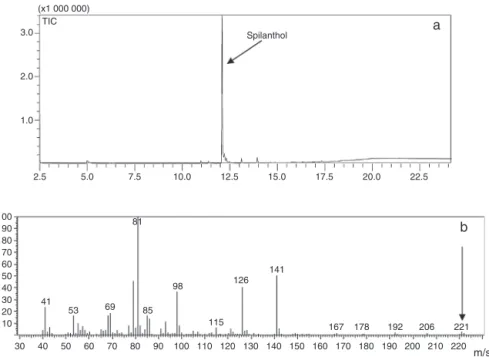
Fig.2.Chromatogramofthedichloromethanefraction(F2R32)(a)andthemassspectrumofspilanthol(b).
model advance III, EUA). Dichloromethane (99.9%, HPLC grade,
Sigma–AldrichInc.,EUA)wasusedassolventintheGC/MS
analy-sis.TheCDCl3(99%,Sigma–AldrichInc.,USA)wasusedassolvent
intheNMRanalysis.TheMEOD(99%,Sigma–AldrichInc.,USA)was
usedassolventintheNMRanalysisofthejambumethanolextract.
TheGC/MSwasequippedwithaFactorFour/VF-5msfused-silica
capillarycolumn(30m×0.25mm×0.25mfilmthickness),using
heliumascarriergasat1ml/min.Theinitialoventemperaturewas
100◦C;itwaskeptconstantfor40minandincreasedattherate
of10◦Cmin−1to290◦C;thefinalisotherm(300◦C)washeldfor
20min.Theinjectionvolumeofthesamplewas1l(1:50split
mode).Boththeinjectorandthedetectorweresetat300◦C.The
massspectrawereobtainedintherangeofm/z10–300,usingthe
electronimpacttechniqueat70eV.Thechemicalcompositionof
thesampleswasanalyzedinaHP5890SeriesIIgaschromatograph
withflameionizationdetector(FID).Thesameoperational
condi-tionsandcolumntypewereusedintheGC/MSanalysis,exceptfor
thetemperaturesoftheinjectorandthedetector,whichwere250
and300◦C,respectively.
Thepercentageofeachcomponentwascalculatedthroughthe
integralareaundertherespectivepeaksincomparisontothetotal
areaofallthecomponentsofthesample.
Themajorcomponentwasidentifiedaccordingtoinformation
fromtheaforementionedanalysismethods and fromdata
gen-eratedthrough thecomparison using1HNMR(Bruker 500MHz
spectrometer)andcarbon13CNMR(Bruker125MHz
spectrome-ter).
Resultsanddiscussion
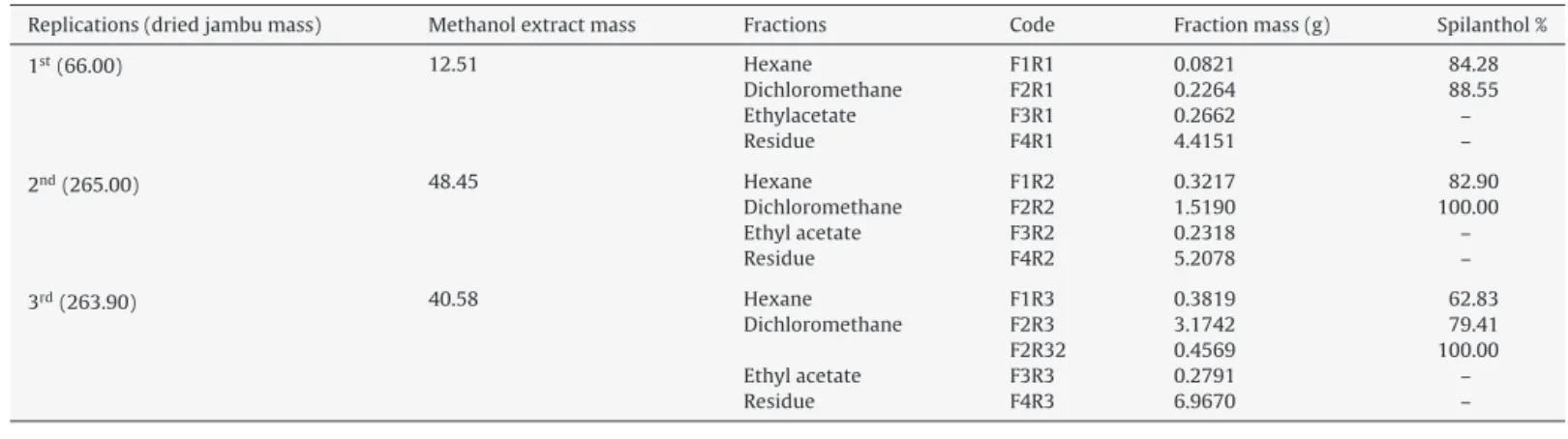
Table1showsthedataoftheA.oleraceamethanolextractand
fractionsfromthreereplications.
Themaincomponentinthehexane(F1R1)anddichloromethane
(F2R32)fractionswasidentifiedthroughthepeaksat12.21and
12.09minintheGCchromatogram(Figs.2aand3).Bothpeaks
pre-sentedmassspectrum,withmainpeaksatm/z(%):221(M+,2),141
(M-C6H8,50),126([C7H12NO]+,40),98([C5H8NO]+,35),81(C6H9+,
100),asitisshowninFig.2b,andthesedatameetthespilanthol
structure.Thespilantholstructurewasalsoconfirmedthrough1H
10 20 30 40 50 60
0.0 1.0 2.0 3.0 4.0
(x1 000 000) TIC
Spilanthol
N-(2-methylbutyl)-(2E,6Z,8E)-decatrienamide
Fig.3. Chromatogramofthejambuhexanefraction(F1R1).
NMRand13CNMRanalysisandthroughitscomparisonwithdata
describedintheliterature(NakataniandNagashima,1992).
Itisimportanttoemphasizethepresenceof
N-(2-methylbutyl)-(2E,6Z,8E)-decatrienamide in the hexane fraction. This amides’
chemicalstructureissimilartothatofspilanthol.Thiscompoundis
alsoknownashomospilanthol,anditisthesecondmostabundant
N-alkylamideintheSpilanthesethanolicextract,sinceitrepresents
9.04%ofthetotalN-alkylamidesamount(Boonenetal.,2010a).
Colorimetryis readilyadaptable toan analysisoftyrosinase
substratesandinhibitors.Substratescanbedeterminedbytheir
enzymatic conversion to darkly pigmented melanin-like
mate-rialswithabsorptionmaximaabout 570p. Inthe presenceof
tyrosine-tyrosinase,inhibitorsofthereactionwillpreventcolour
development(Spenceretal.,1956).
Themechanismofactionoftyrosinaseanditsinteractionwith
l-DOPAwasdescribedindetailbyChang(2009,2012).
Sampleanalysesshowthatthemethanolicextractfromjambu
andthehexanefraction(84.28%spilanthol)areabletoactivatethe
oxidizingactionofthetyrosinaseenzymeforl-DOPA(Table2).
Theincreaseddopachrome formationwasassessed afterthe
directultravioletlightradiationoverthemethanolicextractwas
performedat312nm,anditshowedhighpoweractivationinthe
enzyme.Thiswavelengthispartof theUV-Bradiationwhichis
partlyabsorbedbytheozone intheatmosphere.Theportionof
this radiation that actually reachestheEarth is responsiblefor
skininjuries.Thisanalysiswasperformedattwoconcentrations,
namely: 0.66 and 0.16mgml−1, and it resulted in 57% and 9%
Table1
MethanolextractfractionsfromAcmellaoleracealeaves,stemsandflowerplantmaterial.
Replications(driedjambumass) Methanolextractmass Fractions Code Fractionmass(g) Spilanthol%
1st(66.00) 12.51 Hexane F1R1 0.0821 84.28
Dichloromethane F2R1 0.2264 88.55
Ethylacetate F3R1 0.2662 –
Residue F4R1 4.4151 –
2nd(265.00) 48.45 Hexane F1R2 0.3217 82.90
Dichloromethane F2R2 1.5190 100.00
Ethylacetate F3R2 0.2318 –
Residue F4R2 5.2078 –
3rd(263.90) 40.58 Hexane F1R3 0.3819 62.83
Dichloromethane F2R3 3.1742 79.41
F2R32 0.4569 100.00
Ethylacetate F3R3 0.2791 –
Residue F4R3 6.9670 –
Table2
Tyrosinaseactivationthroughjambumethanolicextractandhexanefraction.
Jambumethanolicextract Hexanefraction
(84.28%spilanthol)
[mgml−1] %ofactivation [mM]a %ofactivation
0.83 357 0.51 40
0.66 297 0.38 23
0.5 107 0.13 10
0.33 63 0.07 5
0.16 39 0.05 1
aRelativespilantholconcentrationinthesamples.
150
120
90
60
30
0 5 10 15 20
0.66 mg/ml 0.16 mg/ml Time in minutes
% activ
ation
25 R2=0.9765
R2=0.9861
30 35
0
Fig.4.TheUV(312nm)radiationeffectontheactivationpowerinjambumethanol
extractonthetyrosinaseenzyme.
impactoftheUVradiationat312nmsignificantlydecreasedthe
poweractivationinthejambumethanolicextract(Fig.4).
Anotherinterestingfactwasobservedduringthekinetic
exper-imentthatusedthehereinadoptedconcentrations.Thepotential
tyrosinaseenzymeactivationthroughhexanefractionat475nm
containing84.28%spilantholreacheditsmaximuminthirtymin;
however,therewasnosignificantpoweractivationdecayupto
60min(Fig.5).
Thedichloromethanefractioncontainingapproximately100%
spilantholshowedtyrosinaseenzymeinhibition(IC50=0.50mM)
duetothehigherenzymeinhibitionpowerwithinthefirst10min.
Afterthistime,theinhibitionpowerdecayed(Figs.6and7).
Intheseexperimentsweobtainedtheincreaseinthepercentage
ofdopachromeproduction,percentageactivation,inadditiontothe
enzymekineticsinthepresenceofthesesamples.
Theabilityofthedichloromethanefractiontoinhibitthe
tyrosi-naseactivitymaybetranslatedintoitspotentialasskinwhitening
agent.Melaninproductionisreducedwhenthetyrosinaseenzyme
activityisinhibited,anditresultsinafairerskin(Karimetal.,2014).
200
150
100
50
0
0 20
0.513 mM 0.385 mM 0.128 mM 0.077 mM 0.051 mM 40
Time in minutes
Absorbance
60 80
Fig.5.Thekineticactionofthetyrosinaseenzymeonl-DOPAinthepresenceof
hexanefraction.
60
50
40
30
20
10
0.13 0.26 0.40
[mM]
% inhibition
0.53 0
Fig.6. Inhibitionactivityofdichloromethane(containingapproximately100%
spi-lanthol)fractiononthetyrosinaseenzyme,withl-DOPAsubstrate.
100
80
60
40
20
0 10 20 30 40
R2=0.999
Time in minutes
% inhibition
50 60 70
0
Fig.7. Kineticsofthetyrosinaseenzymeinhibitionthroughdichloromethane
Theseresultsindicatethatspilantholcanbeusedtocontrolthe
enzymaticbrowninginfruitsandvegetables,sincethisamide
inac-tivatesthetyrosinaseenzyme(polyphenoloxidase)involvedwith
theseproducts’darkeningprocess.Thus,spilantholmayincrease
theshelf liveandthenutritionalqualityofprocessedfruitsand
vegetables,suchasstandardtyrosinaseinhibitorskojicacid,IC50
valueof0.130mM(Okunjietal.,2007).
Tyrosinaseinhibitorsarechemicalagentsabletoreduce
enzy-maticreactions,suchasfruitandvegetable’sskinbrowningand
humanskinmelanization.Therefore,theseagentshavegood
com-mercialpotentialin both thefoodprocessingand thecosmetic
industries(Limetal.,2009).
Conclusion
The activating or inhibitory actions against the tyrosinase
enzyme dependonthe degreeof purity ofjambu extracts and
fractions.Thejambumethanolicextractresultsonthetyrosinase
enzymecorroboratedthehereincarriedoutanalyses,whichused
hexanefractionof84.28%spilanthol.Suchresultprovedthe
tyrosi-naseenzymeactivationthroughdopachromeincrease.Therefore,
jambu-basedproductsmaybeusedascosmetics(creams,soap,
etc.).Atrandom,theycanacceleratemelaninproduction(which
darkenstheskin),aswellasformmelanomas.However,itsuse
againstlocateddepigmentationprocesses(skinandhair)demands
furtherstudies,sincetheanalysisusingUVBirradiationshowed
viableformulationscontainingthisextractintropicalcountries.
Sincethedichloromethanefraction(containingapproximately
100%spilanthol)showedabilitytoinhibitthetyrosinaseenzyme,
spilantholarousesinterestinthestudybasedonformulationswith
this amide in topicalproducts. Surely it canto preventand/or
reduceskinhyperpigmentationprocesses.Anotherpossible
appli-cationofspilantholisthecontrolofenzymaticbrowninginfruits
andvegetables.Themosttyrosinaseinhibitorsarenotcurrently
commerciallyavailable,especiallythosefromnaturalsources,and
thislimitstheirfurtherevaluationinaninvivostudy,whereusually
alargeamountisneededforaninhibitiontest.
Thusfurtherstudiesneedtobeperformedtofoundinhibitors
withahumanclinicalpointofviewincludinghelpandcooperation
ofcosmeticorbiotechnologygroups.
Authors’contributions
AFB,KCS,MCCO,MGC,andAUOSRallcontributedtothe
writ-ingofthisarticle.AFB,MGC,andAUOSRobtainedandidentified
samples.KCSandMCCOperformedenzymaticanalyzes.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
TheauthorsaregratefultoFAPERJ,CNPq,andtoCAPESfor
grant-ingthescholarshipsandthefinancialsupport.
References
Boonen,J.,Baert,B.,Burvenich,C.,Blondeel,P.,deSaeget,S.,deSpiegeleer,B.,2010a.
LC–MSprofilingofN-alkylamidesinSpilanthesacmellaextractandthe transmu-cosalbehaviorofismainbioactivespilanthol.J.Pharm.Biomed.53,243–249.
Boonen,J.,Baert,B.,Roche,N.,Burvenich,C.,deSpiegeleer,B.,2010b.
Transder-malbehaviouroftheN-alkylamidespilanthol(affinin)fromSpilantesacmella
(Compositae)extracts.J.Ethnopharmacol.127,77–84.
Borges,L.da.S.,Vieira,M.A.R.,Marques,M.O.M.,Vianello,F.,Lima,G.P.P.,2012.
Influ-enceoforganicandmineralsoilfertilizationonessentialoilofSpilanthesoleracea
cv.jambuarana.Am.J.Plant.Physiol.7,135–142.
Castro,K.N.C.,Lima,D.F.,Vasconcelos,L.C.,Leite,J.R.S.A.,Santos,R.C.,Neto,A.A.P.,
Costa-Júnior,L.M.,2014.AcaricideactivityinvitroofAcmellaoleraceaagainst
Rhipicephalusmicroplus.Parasitol.Res.113,3697–3701.
Chang,T.-S.,2009.Anupdatedreviewoftyrosinaseinhibitors.Int.J.Mol.Sci.10,
2440–2475.
Chang,T.-S.,2012.Naturalmelanogenesisinhibitorsactingthroughthe
down-regulationoftyrosinaseactivity.Materials5,1661–1685.
Demarne,F.,Passaro,G.2005.UseofanAcmellaoleraceaextractforthebotulinum
toxin-likeeffectthereofinananti-wrinklecosmeticcomposition.USPatentNo.
7,531,193B2.
Dias,A.M.A.,Santos,P.,Seabra,I.J.,Junior,R.N.C.,Braga,M.E.M.,Sousa,H.C.de,2012.
SpilantholfromSpilanthesacmellaflowers,leavesandstemsobtainedby selec-tivesupercriticalcarbondioxideextraction.J.Supercrit.Fluids61,62–70.
Figueiredo,L.C.,2003.Câncerdepele:estudosnosprincipaismarcadores
molecu-laresdomelanomacutâneo.Rev.Bras.Cancerol.49,179–183.
Karim,A.A.,Azlan,A.,Ismail,A.,Hashim,P.,Gani,S.S.A.,Zainudin,B.H.,
Abdul-lah,N.A.,2014.Phenoliccomposition,antioxidant,anti-wrinklesandtyrosinase
inhibitoryactivitiesofcocoapodextract.BMCComplement.Altern.Med.14, 381.
Karioti,A.,Protopappa,A.,Megoulas,N.,Skaltsa,H.,2007.Identificationoftyrosinase
inhibitorsfromMarrubiumvelutinumandMarrubiumcylleneum.Bioorg.Med. Chem.15,2708–2714.
Khan,K.M.,Mughal,U.R.,Khan,M.T.H.,Ullah,Z.,Perveenb,S.,Choudhary,M.I.,2006.
Oxazolones:newtyrosinaseinhibitors;synthesisandtheirstructure–activity relationships.Bioorg.Med.Chem.14,6027–6033.
Ley,J.P.,Krammer,G.,Looft,J.,Reinders,G.,Bertram,H.,2006.Structure–activity
relationshipsoftrigeminaleffectsforartificialandnaturallyoccurringalkamides relatedtospilanthol.Dev.FoodSci.43,21–24.
Lim,T.Y.,Lim,Y.Y.,Yule,C.M.,2009.Evaluationofantioxidant,antibacterialand
anti-tyrosinaseactivitiesoffourmacarangaspecies.FoodChem.114,594–599.
Mbeunkui,F.,Grace,M.H.,Lategan,C.,Smith,P.J.,Raskin,I.,Lila,M.A.,2011.
Isola-tionandidentificationofantiplasmodialN-alkylamidesfromSpilanthesacmella
flowersusingcentrifugalpartitionchromatographyandESI-IT-TOF-MS.J. Chro-matogr.B879,1886–1892.
Miot,L.D.B.,Miot,H.A.,daSilva,M.G.,Marques,M.E.A.,2009.Fisiopatologiado
melasma.An.Bras.Dermatol.84,623–635.
Molina-Torres,J.,Salazar-Cabrera,C.J.,Armenta-Salinas,C.,Ramírez-Sánchez,E.,
2004. Fungistatic and bacteriostatic activities of alkamidesfrom Heliopsis
longipesroots:affininandreducesamides.J.Agric.FoodChem.52,4700–4704.
Molina-Torres,J.,Salgado-Garciglia,R.,Ramirez-Chanez,E.,delRio,R.E.,1996.Purely
olefinicalkamidesinHeliopsislongipesandAcmella(Spilanthes)oppositifolia. Biochem.Syst.Ecol.24,43–47.
Nakatani,N.,Nagashima,M.,1992.PungentalkamidesfromSpilanthesacmellaL.var.
oleraceaClarke.Biosci.Biotechnol.Biochem.56,759–762.
Nascimento,A.M.,deSouza,L.M.,Baggio,C.H.,deP.Werner,M.F.,Maria-Ferreira,
D.,daSilva,L.M.,Sassaki,G.L.,Gorin,P.A.J.,Iacomini,M.,Cipriani,T.R.,2013.
GastroprotectiveeffectandstructureofarhamnogalacturonanfromAcmella oleracea.Phytochemistry85,137–142.
Okunji,C.,Komarnytsky,S.,Fear,G.,Poulev,A.,Ribnicky,D.M.,Awachie,P.I.,Ito,Y.,
Raskin,I.,2007.Preparativeisolationandidentificationoftyrosinaseinhibitors
fromtheseedsofGarciniakolabyhigh-speedcounter-currentchromatography. J.Chromatogr.A1151,45–50.
Oliveira,A.,Amaral,A.J.,Andrade,J.,Nascimento,V.,Mendes,K.,Reis,J.,2011.
Desen-volvendoconstruc¸õesdeapriscosnaagriculturafamiliar.Cad.Agroecol.6,1–4.
Patil,S.S.,Zucker,M.,1965.Potatophenolases.J.Biol.Chem.240,3938–3943.
Ramsewak,R.S.,Erickson,A.J.,Nair,M.G.,1999.BioactiveN-isobutylamidesfromthe
flowerbudsofSpilanthesAcmella.Phytochemistry51,729–732.
Ratnasooriya,W.D.,Pieris,K.P.P.,Samaratunga,U.,Jayakody,J.R.A.C.,2004.Diuretic
activityofSpilanthesacmellaflowersinrats.J.Ethnopharmacol.91,317–320.
Rebello,F.K.,Homma,A.K.,2005.OusodaterranaAmazônia:umapropostapara
reduzirdesmatamentosequeimadas.Amazônia.Cienc.Desenv.1,199–236.
Rios,M.Y.,Aguilar-Guadarrama,A.B.,Gutierrez,M.D.,2007.Analgesicactivityof
affinin,analkamidefromHeliopsislongipes(Compositae).J.Ethnopharmacol. 110,364–367.
Rojas,J.J.,Ochoa,V.J.,Ocampo,S.A.,Mu ˜noz,J.F.,2006.Screeningforantimicrobial
activityoftenmedicinalplantsusedinColombianfolkloricmedicine:apossible alternativeinthetreatmentofnon-nosocomialinfections.BMCComplement. Altern.Med.6,2–7.
Seo,S.Y.,Sharma,V.K.,Sharma,N.,2003.Mushroomtytosinase:recentprospects.J.
Agric.FoodChem.51,2837–2853.
Sharma,V.,Boonen,J.,Chauhan,N.S.,Thakur,M.,deSpiegeleer,B.,Dixit,V.K.,2011.
Spilanthesacmellaethanolicflowerextract:LC–MSalkylamideprofilingandits effectsonsexualbehaviorinmalerats.Phytomedicine18,1161–1168.
Spencer,R.P.,Valentine,R.J.,Field,J.B.,1956.Tyrosinasesubstratesandinhibitors
studiedcolorimetrically.ActaPharmacol.toxicol.12,196–199.
Spiegeleer,B.,Boonen,J.,Malysheva,S.V.,Mavungu,J.D.,deSaeger,S.,Roche,N.,
Blondeel,P.,Taevernier,L.,Veryser,L.,2013.Skinpenetrationenhancing