w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Nanoparticles
containing
curcuminoids
(
Curcuma
longa
):
development
of
topical
delivery
formulation
Cristina
M.
Zamarioli
a,
Rodrigo
M.
Martins
b,
Emilia
C.
Carvalho
a,
Luis
A.P.
Freitas
b,∗aEscoladeEnfermagemdeRibeirãoPreto,UniversidadedeSãoPaulo,RibeirãoPreto,SP,Brazil
bFaculdadedeCiênciasFarmacêuticasdeRibeirãoPreto,NúcleodePesquisasemProdutosNaturaiseSintéticos,UniversidadedeSãoPaulo,RibeirãoPreto,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received12July2014 Accepted3November2014 Availableonline11February2015
Keywords:
Solidlipidnanoparticles Beeswax
Pigskin Curcumin
a
b
s
t
r
a
c
t
SolidlipidnanoparticlesincorporatingCurcumalongaL.,Zingiberaceae,curcuminoidswereproducedby thehotmeltemulsionmethod.ABox–Behnkenfactorialdesignwasadoptedtostudythenanoparticles productionatdifferentlevelsoffactorssuchasthepercentageofcurcuminoids,timeof homogeniza-tionandsurfactantratio.Theoptimizednanoparticleswereincorporatedintohydrogelsforstability, drugreleaseandskinpermeationtests.Theaveragenanoparticlesizeswere210.4nm;thezeta poten-tialof−30.40±4.16;thepolydispersivitywas0.222±0.125.Theaverageencapsulationefficiencyof
curcuminandcurcuminoidswas52.92±5.41%and48.39±6.62%,respectively.Solidlipidnanocapsules
wereobtainedwithcurcuminloadvaryingfrom14.2to33.6%andtotalcurcuminoidsloadashighas 47.7%.ThetopicalformulationcontainingSLN-Curcuminoidsshowedgoodspreadabilityandstability whensubjectedtomechanicalstresstestremainedwithcharacteristiccolor,showednophase separa-tionandnosignificantchangeinpH.Asaresultofslowrelease,thenanoparticleswereabletoavoid permeationorpenetrationinthepigearepidermis/dermisduring18h.Thetopicalformulationis sta-bleandcanbeusedinfurtherinvivostudiesforthetreatmentofinflammatoryreactions,inspecialfor radiodermitis.
©2014SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
InthelastdecadethecurcuminoidsfromCurcumalongaL., Zin-giberaceae,werereportedinseveralmolecularsignalingpathways ofclinicalimportance(Goeletal.,2008;Dasetal.,2010;Mourtas etal.,2011).Theyhaveanti-inflammatory,antifungal, antimicro-bial,antioxidant and antiproliferative properties (Aggarwal and Harikumar,2009;Augustyniaketal.,2010;Irvingetal.,2011;Zhou etal.,2011),amongothers.ThemaincurcuminoidsfromCurcuma arecurcumin,desmethoxycurcuminandbisdesmethoxycurcumin, threebioactivesubstancescommonlyusedasspicesandcoloring agentsinfood.However,thesemoleculeshavepoorbioactivityand instabilityinneutralandalkalineaqueoussolutions(Tønnesenand Karlsen,1985;Yallapuetal.,2012)andhydrophilictopical formula-tions.Besides,whencurcuminoidsweresubjectedtolightexposure therewas30%degradationand1.8%lossunderoxygenactionin30 days.Despitetheirseveralutilitiesandlowtoxicity(Jayaprakasha etal.,2006),thecurcuminoidslowbioavailability(Gaoetal.,2011)
∗ Correspondingauthor.
E-mail:lapfrei@fcfrp.usp.br(L.A.P.Freitas).
contributestorecentgrowthofinterestindevelopingnewcarrier systems(Nairetal.,2010).
Duetoanatomicalandphysiologicalcharacteristicsoftheskin, someingredientsmaynotacquirethedesiredactivitytopicallyand newdrugcarriersystemshavebeenusedtomodifythe perme-ation/penetration.AccordingtoMehnert andMader(2001),itis becomingevidentthatthedevelopmentofasingledrugisnolonger sufficientfortheevolutionoftherapy,giventheinsufficient con-centrationofthedrugduetoitslowabsorption,rapidmetabolism andelimination;hightoxicity incombinationwithotherfabrics andhighfluctuationinplasmalevelsduetounpredictable bioavail-abilityafterperioraladministration.
Lipid-basednanoparticlesystemsarecommonlyusedin topi-calapplications(Contrietal.,2011)andarepreparedwithsolid structuresatroomtemperatureoratbodytemperatureassociated withsurface-activeagentsandwaterlipids.AccordingtoOliveira (2008),lipophilicdrugswillhaveaneasierpenetration,giventhe characteristicsoftheplasmamembrane.Inthisregard,skin per-meationenhancingagentscanbeusedintheformulationsandcan reduceskinresistance(MartinsandVeiga,2002).
Over the past 10 years solid lipid nanoparticles (SLN) and nanostructuredlipidcarriers(NLC)throughmixturesoflipidsthat
http://dx.doi.org/10.1016/j.bjp.2014.11.010
acquire a specific molecular arrangement were produced, suf-feringa majoreffectofsurfactantsadded totheseformulations (Serraetal.,2009).Thiscombinationofsurfactantsisconsideredto improvestabilitybypreventingagglomerationoftheparticlesand retardingthetimeofpolymorphictransitionoflipidsto encapsu-latetheactiveingredient(Serraetal.,2009).
Hydrophilicmatrix systems are commonly used due to low productioncosts,acceptancebyregulatoryorgan,simplicityof for-mulation,productionfacility, capacitytoincorporatearange of substanceswithextendedsolubilityrange(Lyraetal.,2007),andin general,haveathixotropicorpseudoplasticrheologicalbehavior, duringapplicationbecomingmorefluid,facilitatingthespreading andrecoveringtheinitialviscosityuponendingtheapplication, whichpreventstheproductdrips(Martin,1993).Theseproducts tendsto have a longershelf life, becauseduring storage (dur-ingwhich time theproductremains at rest),presents constant viscosity,whichmakesdifficulttoseparatingtheformulation con-stituents.
Hydrophilicgelshavebeendemonstratedtobeadequatefor incorporation of SLN (Jenning et al., 2000). By reducing trans-epidermallossofwaterandimprovingskinhydration,anincrease in penetration and percutaneous absorptionhas beenreported (Jenningetal.,2000;Wissingetal.,2001).Otheradvantageofthis systemistopromotethecontrolledreleaseofsubstances, reduc-ingtheirsystemicabsorptionandactingasaphysicalsunscreen (AttamaandMuller-Goymann,2008).Thesepropertiesareofgreat importancefortheprotectionofskinfrompatientsundergoing radiotherapytreatment.
Theaimofthisresearchwastodevelopsolidlipidnanoparticles (SLN)containingcurcuminoidsusinga naturalwaxas encapsu-latingmatrix andtodevelop andcharacterizeahydrophilicgel, fortopicalapplication.Thenanoparticlepreparationwasstudied applyingaBox–Behnkendesignandthehydrogelstabilityandex vivoskincurcuminoidpermeation/penetrationwasevaluated.
Materialsandmethods
Plantandothermaterials
ThecurcuminoidconcentratewaspurchasedfromCHR (Chris-tianHansenA/S,Denmark)andpharmacognosticallycharacterized, andasamplecounterproofwasdepositedatthe“Herboteca LADI-FARP”fromtheFCFRP/USPwithRegistrynumberCP01/14-S.The powdermoisturecontent,total ashcontent,swellingindex and sizedistributionweredetermined accordingtothe methodolo-giesdescribedinFarmacopeiaBrasileira(2010).Theresultswere expressedasmean±standarddeviationofthreereplicates.
Analyt-icalgradecurcuminasastandardforquantificationpurposewas purchasedfromSigma(Sigma–Aldrich,St.Louis,MO,USA).
Quantificationofcurcuminandcurcuminoids
Forcurcuminandtotalcurcuminoidcontentdetermination a HPLCmethodwasdeveloped.Briefly,anUltimate3000(Thermo FisherBrasilLtda,São Paulo,Brazil) HPLCwasusedwitha C18 Alcromcolumn(PhenomenexLtda,SãoPaulo,Brazil).Themobile phasewas50:50acetonitrileandacidifiedwater(1%citricacid)at pH3.5,flowrateof1.0ml/min.TheUV–visdetectorwassetupto 425nm.50lsampleswereinjected(Pauluccietal.,2013).
Thecurcuminstandard (Sigma–Aldrich, St.Louis,USA) solu-tion was prepared with 1mg of curcumin in 5ml of 50:50 acetronitrile:water (1% citric acid). Consecutive dilutions were made to obtain seven solutions at concentrations from 25 to 2500ng/ml,whichwereinjectedtopreparetheanalyticalcurve. Themethodlinearityandselectivitywerealsoevaluated.Samples
ofdrug(curcuminoids),unloadednanocapsules,curcuminloaded nanocapsules,pigearextract,hydrophilicgel,andgelcontaining nanocapsulesweretestedforselectivity.
PreparationofsolidlipidnanoparticlesSLN
SLNwerepreparedbyhotmeltemulsiontechnique(Martins, 2014; Zamarioli, 2014) employing a high-shear homogenizer. Briefly,waterwasheatedat70◦CwithTween80(polyethylene gly-colsorbitanmonooleate)andlecithin(phosphatidylcholine),prior toadditionoftheoilyphase.Thebeeswaxandcurcuminoidswere heatedandhomogenizedatsametemperature.Themicroemulsion wasproducedbydroppingtheoilyphaseinaqueoussolutionwith theaidofaglasssyringe.Thedispersionwassubmittedtohighshear homogenizationat70◦CusinganUltra-TurraxT25(IKA®, Wilming-ton,USA)at20,000rpmduring20min.Thelipidnanoparticleswere solidifiedbycoolingdownthedispersion.Allexperimentswere keptinanicebathwithmagneticstirringafterhomogenizationfor 10min.
Factorialdesign
TheSLNproductionwasstudiedusingaBox–Behnkenfactorial design(Boaxetal.,1978)forthreefactorsatthreelevels, result-inginfifteenexperiments.Table1showsthestudiedfactorsand therangesofeachone:thepercentageofcurcuminoidsinbeeswax nanoparticles,%C:Bw;thehomogenizationtimeusingTurrax,HT andtheweightproportionoflecithintoTween80,L:T.Thechoices oftherangesofthefactorsshowninTable1werebasedon prelim-inaryexperimentsandtherangeofproportionoflecithintoTween 80wasbasedonthehydrophilic–lipophilicbalance(HLB).
CharacterizationofSLN
Theparticlesize(Dp)distributionandpolydispersivityindex (PdI) were obtained by photon spectroscopy (PCS) employing a Zetasizer Nano Series (Malvern Instruments Limited, Worcs, UK). Zeta potential was assessed by determining the particle electrophoreticmobility using thesameapparatus. These mea-surementswereperformedat25◦Cusingwaterasthedispersing mediumanddilutionofsuspensionsofnanoparticlesataratioof 1:100inultrapurewater(MilliQ,MilliporeInc.,USA).
Morphology
ThesurfaceappearanceandshapeofSLNweredeterminedby anatomicforcemicroscope(AFM)modelSPM-9600(ShimadzuCo., Kyoto,Japan).ThedispersionofSLNwasdiluted(1:100)using ultra-purewater(Milli-Q,MilliporeInc.,USA)andspreadontothinmica plates.
Encapsulationefficiencyanddrugload
Table1
Levelsandcodificationofvariablesstudied.
Factors Level(−1) Level(0) Level(+1)
X1–percentageofcurcuminoids/beeswax 5% 10% 15%
X2–timeinTurrax*inmin 10 15 20
X3–proportionofLecithin/Tween80 0.5 1.0 1.5
to a cycle of centrifugation at 9300×g for 20min. The super-natantwasaspirated,filteredandplacedinvialsforcurcuminand curcuminoidquantification.Thelatterwereconsideredtobethe curcuminoidsthatwereencapsulatedintheSLN.Theencapsulation efficiency,%EE,wascalculatedbyEq.(1).
%EE=(CC/CT+CC)×100 (1)
whereCEncistheamountofcurcuminoidsinsidetheSLNandCT isthetotalamountofcurcuminoidsaddedtotheformulation.
Preparationoftopicalformulation
Inordertocontemplatetheobjectiveofthisproject,wechoseto prepareaknownbaseformulation,thegelNatrosol®(ViaPharma Ltda,SãoPaulo,Brazil)ataconcentrationof1.5%.Theincorporation ofSLNdispersionsintogelcomponentswasperformedatroom temperature,asbeeswaxbecomessoftenedataround50◦C.
Invitroreleaseprofile
Briefly,analiquotof20loftheSLNdispersion correspond-ingto59.2mgofcurcuminoidswasdispersedinphosphatebuffer, distributed in fivetubes and keptatroom temperature.Atthe predeterminedintervals,thetubeswerecentrifugedat200×gfor 5min(Bishtetal.,2007)toseparatethecurcuminoidscrystals pre-cipitatedafterreleasefromSLN.Aftercentrifugation,thedispersion wasaspiratedandplacedintonewtubesforsubsequent centrifu-gation,andagainthecurcuminoidremainingcrystalsdissolvedin 1mlofmethanol.Thisvolumewasfiltered(0.45M)andanalyzed inHPLCforquantification.
Exvivopermeationstudy
ThepermeationtestswereperformedusingFranzcell diffu-sion(1.77cm2 of area) withpigear skin.Thepig earskinwas obtainedfromslaughterhouseoverseenbytheMinistryof Agri-culture,locatedinthemunicipalityofIpuã(OlhosD’ÁguaIndústria eComérciodeCarnesLtda,Ipuã,SP,Brasil).
Theexperimentswereperformedwithsixcells:(1) onecell forthecontrol groupwithcurcuminoids solution;(2) two cells withNatrosolgelcontainingcurcuminoidsand(3)threecellswith thegelcontainingnanoencapsulatedcurcuminoids(0.5gofgelin each).Forthereceptorsolution0.2Mphosphatebufferwasused with20%ethanol(Suwantongetal.,2007),pH7.5,maintainedat 37±0.5◦Cinthermostaticbathwithcontinuousstirringfor18h. Sampleswerewithdrawnfromthereceptorcompartmentafter0, 15,30and60minandat2,4,6,8,10,12,14,16and18hofassay andimmediatelyquantifiedbyHPLC.
Afterthe18h, theskinwasremoved fromFranzapparatus, dried with a fine cloth and repetitive (n=15) removal of the stratumcorneumwasperformedwithadhesivetape(3M,Sumaré, SãoPaulo,Brazil)19mm×50m.ThetapeswereplacedinFalcon
tubes(50ml)containing0.2Mphosphatebufferwith20%ethanol (Suwantongetal.,2007),agitatedinavortexmodelAT56(Phoenix) for3min,filteredthrougha0.45Mmembrane(Millipore Corpo-ration,Billerica,MA,USA)andquantifiedinHPLC.
Retentionintheepidermisanddermis
TheepidermisanddermisweremincedandplacedinFalcon tube(50ml)containing0.2Mphosphatebufferwith20%ethanol (Suwantongetal.,2007),subjectedtohighshearagitation(Turrax) at3000rpmfor1min;sonicatedinanultrasoundbathModelUSC 1400(UltrasonicCleaner)for30minandcentrifugedfor10minat 2790×ginacentrifugemodelHT–MCD2000(BiosystemsLtd., Curitiba,Brazil).Thesolutionobtainedwasfiltered(0.45M)and quantifiedinHPLC.
StabilityofgelformulationwithSLN
Preliminarily,theNatrosol®gelcontainingSLN-Curwas sub-mitted tocentrifugation at 2790×g for 30min (Anvisa, 2004). Following,theformulationwassubjectedtothermalstresscycles alternating24hintheovenat40±2◦Cand24hintherefrigerator at 5±2◦C. Attheend of each cycle, organolepticand physico-chemicalcharacteristicsofthegelwereevaluated.ThepHofthe sampleswasobtainedusingaDM20pHmeter(Digimed,SãoPaulo, SP,Brazil).Thisstudywasperformedinsixcompletecycles.Also, organolepticandpHevaluationofgelcontainingtheSLN-Curwas performedafterthreemonthsofstorage.Sampleswerekeptattwo testtemperatures(25±2◦Cand5±2◦C)bothintheabsenceand presenceoflight.
Statistics
ToexaminethisplanMinitab16software(MinitabInc.,State College,PA)wasused,withitsfeaturesof descriptivestatistics, experimentaldesign(DOE)andresponsesurface;togeneratethe response surface graphs, Statistica version 12 (StatSoft, South America,SãoCaetanodoSul,SãoPaulo,Brazil)wasused. Descrip-tive analyses were mean, standard deviation, minimum, and maximum.Confidenceintervalwasestablishedfortheanalysisof thereleaseprofileofcurcuminoidsfromSLNtakenandvariance analysisinallexperimentaldesigns,withp<0.05.
Resultsanddiscussion
The drug raw material was characterized according to FarmacopeiaBrasileira(2010)withallassaysmadeintriplicate. Thetotalashcontentwas0.10±0.01%,andmoisturecontentwas
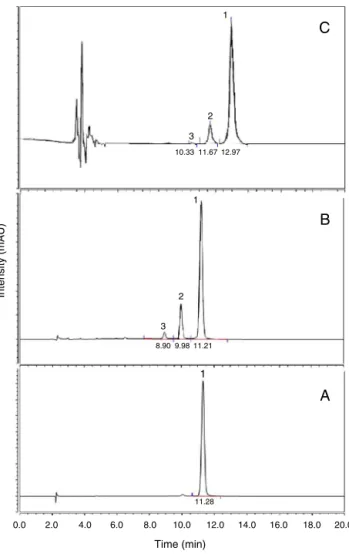
0.75±0.02%.MeanparticlesizeD50was30.8m,whileD10and D90were13.7and168.2m,respectively.Sizedistributionspan was0.20.Thecontentsofcurcumin,demethoxycurcuminand bis-demethoxycurcumininthedrugweredeterminedbyHPLCusing thecurcuminstandardfromSigmaandshowedtobe76.7,19.4and 3.9%,respectively.
0.0
8.90 3
Intensity (mAU) 2
1
9.98 11.21
11.28 1 10.33
3 2
A
B
C
1
11.67 12.97
2.0 4.0 6.0 8.0
Time (min)
10.0 12.0 14.0 16.0 18.0 20.0
Fig. 1.Chromatograms obtained for: (A) Curcumin analytical standard (Sigma–Aldrich); (B) drug containing the three curcuminoids used as raw material;(C)sampleextractedfrompigskinafterpenetration/permeationtest.
TheBox–Behnkendesignwasidealizedafteraseriesofprevious experiments, varying the concentrations of beeswax, curcumi-noids, surfactants, temperature, duration of shearing, mode of curcuminoid addition and others. The results showed that the HLB(hydrophilic/lipophillicbalance)isveryimportantduringthe nanocapsulespreparationandwasconsideredinthefurtherwork. TheBox–Behnkendesign appliedcanbeseenin Table1,which showsthefactorsstudiedandtheirlevels.Theresultsof nanoparti-clesizes,polydispersivity(PdI)andzetapotentialforallthefifteen experimentalconditionsareshowninTable2.Ascanbeseenin Table2,thePdIvariedwidelyfrom0.128to1.000.HighvaluesofPdI mayinterferewiththereliabilityoftheestimatedsizeofparticles (Zetasizer,2009).TheMalvernZetasizer(2009)softwareapplies thecumulativeanalysismethod,andcalculatesthezeta-average (size)andpolydispersivityfromthecoefficientsofthevirial equa-tion.Whencumulativeanalysisisnotapplicable,thePdIvaluesare above0.100andneitherzeta-averagenorPdIvaluesarereliable. Basedonthis,thediameterswereextracteddirectlyfromthe fre-quencydistributionofsizes,i.e.,fromthecentervalueofthepeak withhigherintensity.
ThreeexamplesofthesizedistributionscanbeseeninFig.2. Fig.2Apresentsthesignalintensitydistributionforexperiment2 fromTable1,wheretheSLNwaspreparedwith15%of curcum-inoids,20minathighshearhomogenizerandratiooflecithinto Tween80equalto1.0.Asshowninthisplot,thereisonlyonepeak withareasonablenarrowdistributionandsizesvaryingfrom100to 200nm.Dependingontheconditionsofpreparationtheprofileof
thedistributioncanchangeconsiderably,asseeninFig.2B,which showsthesizedistributionforexperiment5fromTable1.Inthis processingconditiontherearetwowelldefinedpeaks,orabimodal distribution:thefirstpeakwithsizefrom20to80nmandthe sec-ondandlargerpeakwithsizefrom100to600nm.Inthiscondition, thereisalsoaverysmallpeakappearingatsizesabove7000nm. Fig.2Cshowsathirdinterestingresultinaunimodaldistribution showingsizesfrom150to550nm(experiment11inTable1).These threeplotsareveryillustrativeofthestrongeffectofexperimental conditionsonthenanoparticlessizedistributions,andthe impor-tanceofusingmultivariatetoolsforeffectselucidation.Sinceour analysismustbebasedontheintensitydistribution,onlythelargest peaksweretakenintoconsiderationformeansizedetermination atthesituationswheretherearetwoormorepeaks.
Thevaluesofmeansizes,polydispersivityandzetapotentialfor allfifteenexperimentsareshowninTable2.Consideringall exper-imentsinTable2,anaveragedparticlesizewas210.4±146.6nm,
withminimumandmaximumof32.7nmand481.9nm, respec-tively. Theaveraged zetapotentialwas−29.0±4.2mV,varying
froma minimum of -35.3mVto a maximumof −21.2mV.The
averagedPdI was0.546±0.300,witha maximumof 1.000and
minimumofthe0.128,butonemustrememberthatthisPdIwas calculatedbyMalverncumulativeanalysisandcanonlybeused forqualitativepurposes(Zetasizer,2009)andnotforquantitative comparison.
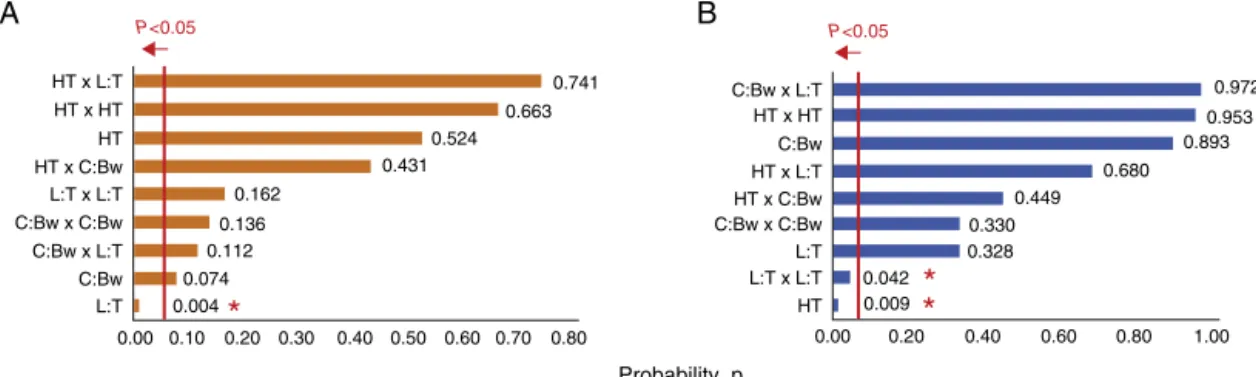
Theanalysisofvariance,ANOVA,byresponsesurface methodol-ogyresultedinafewsignificanteffectsofthefactorsstudiedonDp, PdIandZP.TheParetoplotsinFig.3bringtheprobabilitiespforall thelinear,quadraticandinteractioneffectsfortheBox–Behnken designs.Fig.3AshowstheParetoplotforDp,whichindicatesthat onlythelineareffectoftheratiooflecithin/Tween,L:Twas sig-nificanttoSLNsizesat5%(p<0.05).Theotherfactorsand their interactionswerenotsignificantwithintheconfidenceintervalof 95%.Thepolynomialequationtofitthesurfaceresponsehasonly X3asavariable,aspresentedinEq.(1).
Dp=144.5+143.6(X3) (1)
Theeffectofsurfactantsratio,L:T,onDpwastheunique signif-icanceincurcuminoids/beeswaxSLN.Otherauthors(yetforJebors etal.,2010)reportednoinfluenceofagitationspeedonthe parti-clesize,asobservedinthiswork,andthiscanbeattributedtothe thermodynamiceffectofsurfactantadditionasbeingprimordial inemulsionformation.Besides,theuseofsmallglasswareinthe labscaleiscommonlyassociatedtostrongwalleffects,whichmay causethemultimodal sizedistributions,whichwasobservedin Fig.2B,andfourotherexperimentalconditionsinthiswork.A com-monpracticeistofilterthesamplestocutoffundesiredsizesusing 0.22and0.45mmfilters.Inthiswork,however,thedecisionforno filtrationaimedtostudytheactualimplicationsofthemethodof productionandtherealinterferenceofthefactorsanalyzed.
ForthePdIonlythelineareffectsof%C:Bw(X1)andL:T(X3)were significantat theadoptedsignificance level(p<0.05).Theother factorswerenotsignificantwithintheconfidenceintervalof95%, sotheywerenotkeptinthepolynomialfittingpresentedinEq.(2).
PdI=0.398+0.201(X1)−0.309(X3) (2)
ThechangesinlecithinconcentrationwhenL:Tisvariedmaybe relatedtoincreasedpolydispersityoftheparticles,sincethereisa possibleformationofmultiplelayersoflecithinonthesurfaceof theSLNortheformationofothercolloidalstructures(Jeborsetal., 2010).
Table2
Size,PdIandzetapotentialofSLN-Cur.
Exp. %Curcuminoids/beeswax TimeinTurrax Proportionlecithin/Tween80 Size(nm) Peakintensity(%) PdI Zeta(mV)
1 5 20 1.0 405.5 95.1 0.429 −21.2
2 15 20 1.0 128.9 100.0 0.627 −21.4
3 5 10 1.0 201.2 91.5 0.373 −28.7
4 15 10 1.0 62.2 100.0 1.000 −33.4
5 5 15 0.5 247.6 82.7 0.543 −33.3
6 15 15 0.5 45.0 100.0 1.000 −31.3
7 5 15 1.5 374.9 100.0 0.179 −28.9
8 15 15 1.5 481.9 100.0 0.504 −27.1
9 10 20 0.5 32.7 100.0 1.000 −29.1
10 10 10 0.5 62.4 100.0 0.972 −34.0
11 10 20 1.5 297.0 99.0 0.236 −28.0
12 10 10 1.5 382.9 100.0 0.128 −35.3
13 10 15 1.0 159.4 100.0 0.461 −29.3
14 10 15 1.0 150.2 100.0 0.361 −25.0
15 10 15 1.0 123.9 100.0 0.371 −29.1
A
B
C
0 0 0
5 10 15 20 25
2 4 6 8 10 12 14
10
10
Intensity %
100 1000 10000 10 100 1000
Size (nm)
10000 10 100 1000 10000
20 30 40
Fig.2. Sizedistributionbyintensityofexperiments2,5and11(A–C).
withintheconfidenceintervalof95%,sotheywerenotkeptinthe polynomialfitpresentedinequation,
ZP=−27.80+3.96(X2)−3.89(X3)2 (3)
The magnitude of the ZP can be associated with the ratio of lecithin usedin theformulations and interactionwithother components such as the Tween, which is a non-ionic surfac-tant.
Encapsulationefficiency
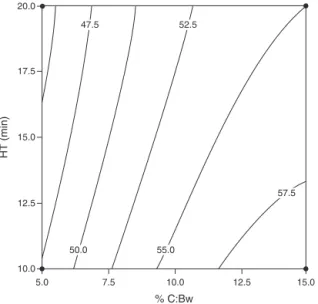
Theencapsulationefficiency,EE,variedfrom41.4to62.0%for curcuminandfrom31.0to58.78%fortotalcurcuminoids.The aver-agedcurcuminencapsulationefficiencyforthefifteenexperiments was52.92±5.41%andfortotalcurcuminoids,48.39±6.62%.The ANOVAbyresponsesurfacemethodologyshowedthatonly%C:Bw affectedEEatthesignificancelevelof5%.Fig.4showsthecontour plotofcurcuminencapsulationefficiencyinbeeswaxnanoparticles asafunctionofhomogenizationtime,HT,andpercentcurcumin
content,%C:Bw.Althoughliteraturedata(Yallapuetal.,2013) indi-catethatcurcuminencapsulationcanbehigherforothercarriers, EEabove50%maybeconsideredgoodforalipophilicdrugandthe EEvariesstronglydependingondifferentsystemsandprocesses. Apolynomialequationwasfittedtothedateof%EEforcurcumin andisgiveninEq.(4).
%EE=53.30+5.60(X1) (4)
Morphologicalanalysis
Fig. 5 shows the morphology of the SLN by atomic force microscopy, AFM.Fig. 5A presentsthetopographic view,while thefrontalviewisshowninFig.5B.Itwasevidentthatparticles weresphericalinshapeandhomogeneouslydistributedwithsize rangedbetween52nmand101nm,smallerthanthatmeasuredby dynamiclightscattering.
A
B
P<0.05 P<0.05
HT x L:T
HT x L:T
HT x HT HT x HT
L:T x L:T
L:T x L:T HT x C:Bw
HT x C:Bw
C:Bw x C:Bw C:Bw x C:Bw
C:Bw x L:T
C:Bw x L:T
C:Bw
C:Bw
L:T
L:T
0.00 0.10 0.004
0.074 0.112
0.136 0.162
0.431 0.524
0.663
0.009 0.042
0.328 0.330
0.449 0.680
0.893 0.953
0.972 0.741
*
*
*
0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.00 0.20 0.40 0.60 0.80 1.00
HT
HT
Probability, p
5.0 10.0 12.5 15.0
HT (min)
17.5 20.0
7.5 10.0 12.5 15.0
% C:Bw
50.0 55.0
57.5 52.5
47.5
Fig.4.Contourplot ofencapsulationefficiency,EE%,ofcurcumininbeeswax nanoparticlesasafunctionofhomogenizationtime,HT,andpercentcurcumin content,%C:Bw.Onlytheeffectof%C:Bwissignificantatp<0.05.
Releaseprofile
Allthesamplesofnanocapsulestestedshowedasustainedbut notlinearrelease(Fig.6),asseenintheliteratureasafirstorder kinetics(Nayaket al.,2010; Pugliaet al.,2012).Approximately 50–60%ofcurcuminwasreleasedafter2hofexperiment. How-ever,around40%ofcurcuminoidsremainedunreleasedafter10h ofexperiment.Nayaketal.(2010)found,usingthesame method-ology,thatafter12hofexperiment70%ofcurcuminoidshadbeen released,andwithin24hofreleasewasaround80%.The extended-releaseprofilemayindicateinteractionsbetweenthelipidandthe drugandamorecentrallocationofthisdrugintheSLN(Küchler etal.,2009),beyondtheinfluenceofthesurfactantsused.
Preliminarystability
Theformulationdidnotundergophaseseparationwhen sub-jectedtomechanicalstresstestbycentrifugation(Anvisa,2004). Thegelremainedhomogeneous,withbrightandlightyellowcolor, characteristicodorandnophaseseparationduringthemechanical stressat3000rpmfor30min.Fig.7showsthepHvaluesmeasured attheendofeachthermalstresscycleof24hat40◦Cand24hat 5◦C.ThepHremainedprettystableoverthetestperiod(Fig.8)and ithasundergoneminorchangesthatwerenotsignificantly differ-entthanthepHofthegelinitiallymade.ThepHofthegel,slightly below6.0,isphysiologicallyadequatefortopicaluseandalso ade-quateforchemicalstability,sincecurcuminoidsareunstableatpH
0 20 30 40 50 60 70 80
0.5 1 2 3 6 12 16
Time (h)
% curcumin released
Fig.6.Curcuminoidreleaseinphosphatebuffer(pH7.4)solutionafter16-htest. Experimentsmadeinsextuplicate.
0.0
pH (-)
Cycles (48 h)
1st 2nd 3rd 4th 5th 6th
1.0 2.0 3.0 4.0 5.0 6.0
Fig.7. pHofhydrogelcontainingthecurcuminSLNbasedonpHvaluesaftersix 48hstresscyclesofheating(40◦Cfor24h)andcooling(5◦Cfor24h).
above7(TønnesenandKarlsen,1985;RusigandMartins,1992). Anotherstabilitytestwasrunfor90days,withgelsamplesstored attesttemperaturesof25±2◦Cand5±2◦C,intheabsenceor pres-enceoflight.Theorganolepticevaluation ofthegelshowedno significantchangesinappearancesandnophaseseparation.The pHofthegelcontainingtheSLN-Curdidnotchangesignificantly afterthreemonthsifcomparedtothebeginningofthetests.The pHwas5.48forthegelkeptat25±2◦Cinabsenceoflight,5.13for
thesampleatthesametemperaturebutunderdaylightand5.66for thegelstoredat5±2◦C.Thecentrifugationtestwasalsoperformed andthethreesamplesdidnotundergophaseseparation.
Exvivopenetrationandpermeationstudy
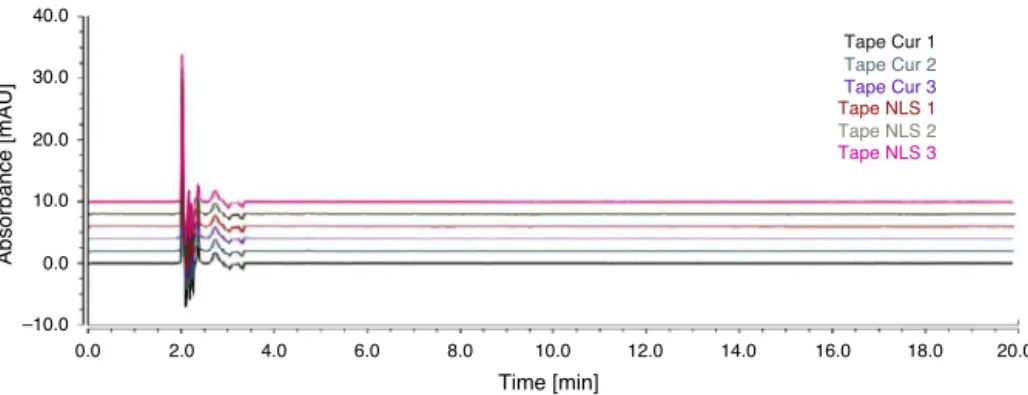
TheHPLCanalysesofextratumcorneumandepidermis+dermis afterthe18hexperimentsinFranzcellsareshowninFigs.8and9, respectively.Therewasnopermeationofcurcuminoidsinpigear skin during any of the sixplicates, which indicate a controlled releasefromSLNingelwithreleasetimelongerthan18h.Due
A
B
1.00
1.00
0.50 0.50
0.00
40.0
30.0
20.0
10.0
0.0
Absorbance [mAU]
–10.0
0.0 2.0 4.0 6.0 8.0 10.0 12.0 14.0 16.0 18.0 20.0
Tape NLS 3
Tape NLS 2
Tape NLS 1 Tape Cur 3 Tape Cur 2
Tape Cur 1
Time [min]
Fig.8. Chromatogramsobtainedfromsamplesofextratumcorneumafterthepenetration/permeationtests.Aimingforquantificationofcurcuminoidsaftertapestripping.No peaksofcurcuminoidsweredetected.(Note:TriplicatetapesoftheskinexposedtothegelwithfreecurcuminoidsandtapeoftheskinexposedtogelwithSLN-curcuminoids.)
0.0 –10.0
–5.0 0.0 5.0 10.0 15.0 20.0
Absorbance [mAU]
2.5 5.0 7.5 10.0
Time [min]
12.5 15.0 17.5
Epi/Der NLS 3 Epi/Der NLS 2 Epi/Der NLS 1
Epi/Der Cur 3 Epi/Der Cur 2 Epi/Der Cur 1
20.0
Fig.9.Chromatogramsobtainedfromsamplesofpigearskinafterthepenetration/permeationtests.Aimingforquantificationofcurcuminoidsinepidermisanddermis. Nopeaksofcurcuminoidsweredetected.(Note:TriplicatetapesEpidermis+dermisexposedtothegelwithfreecurcuminoidsandepidermis+dermisexposedtogelwith SLN-curcuminoids.).
tolimitations imposedbytheuseofexvivobiologicalmaterial, thepermeationexperimentcannotbelongerthan18h,sincethe pigearskinundergoesstrongchangesafterthisperiodat37◦C (Sartorellietal.,2000).
Penetrationofcurcuminorcurcuminoidswasnotobservedfor anyformulationintriplicate,e.g.,gelcontainingtheSLN-Curandgel withfreecurcuminoids,whichcorroborateswiththeideaofvery slowreleasefromnanocapsules.Theresultscorroboratethe state-mentthatnanotechnologycanmodifythepenetration/permeation ofsubstanceandcontroltheirrelease,increasingtheresidencetime ontheskinsurface(Luengoetal.,2006;Guterresetal.,2007;Cevc andVieri,2010).
Sincethegoalofthisworkwastodevelopananti-inflammatory topicalformulationforradiodermitis,itisadequatethat curcumi-noidsshowaslowreleaseandthatSLN-Curdoesnotpenetratein normalskin,behaviorthatcanbesignificantlychangedinskin dam-agedbyradiotherapybuns.Theresultisstimulatingandshouldbe confirmedinfutureinvivotestsfornormalandinjuredskin,since penetration/permeationinvivocanbeconsiderablydifferentfrom exvivobecauseofshearcausedbybodymovementandalsoby skininjurescausedbyradiation(radiodermitis),whichmayfavor thepenetrationofcurcuminoids.
Conclusions
Beeswax showed tobe an excellent natural carrier for cur-cuminoids nanoencapsulation, resulting in nanocapsules with adequatesizeand zetapotential.Encapsulationefficienciesand drugloadinnanocapsulesalsodemonstratethattheprocess devel-oped is promising. The application of factorial design tostudy
the preparation process for nanocapsules showed tobe a very importanttool,allowingestablishingtherelationshipsamongthe factorsandqualityattributes.Hydrophilicgelcontainingsolidlipid nanocapsulesshowedtobestableinpreliminarytestsandthat pen-etration/permeationinpigskincouldnotbedetectedin18h.Assay inFranzcellsusingpigearskinshowednopenetration/permeation ofnanoencapsulatedcurcuminoids.Theresultsarestimulatingfor futureinvivotestsofanti-inflammatoryactivityinradiodermitis treatment.
Authors’contributions
CMZ(MScstudent)wasresponsibleformostofexperimental work(nanocapsules,gel andcharacterization); RMMdeveloped thenanoencapsulationtechniqueandcontributedinalllaboratory workandchromatographicanalysis.ECCidealizedtheworkand contributedtocriticalreadingofthemanuscript.LAPFdesigned thestudy,supervisedtheexperiments,criticallyreadanddefined thefinalversionofthemanuscript.Alltheauthorshavereadthe finalmanuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
References
Aggarwal,B.,Harikumar,K.B.,2009. Potentialtherapeuticeffectsofcurcumin, theanti-inflammatoryagent,againstneurodegenerative,cardiovascular, pul-monary,metabolic,autoimmuneandneoplasticdiseases.Int.J.Biochem.Cell Biol.1,40–59.
Anvisa,2004.GuiadeEstabilidadedeProdutosCosméticos.In:MinistériodaSaúde. AgênciaNacionaldeVigilânciaSanitária,1sted.Anvisa,Brasília(SérieQualidade emCosméticos,1).
Attama,A.A.,Muller-Goymann,C.C.,2008.Effectsofbeeswaxmodificationson thelipidmatrixofsolidlipidnanoparticlescrystallinity.ColloidsSurf.A315, 189–195.
Augustyniak,A., Bartosz,G.,Cipak,A.,Duburs,G.,Kov L’Ubica, H.,Luczaj,W., Majekova,M.,Odysseos,A.D.,Rackova,L.,Skrzydlewska,E.,Stefek,M.,Strosov, M.,Tirzitis,G.,Venskutonis,P.R.,Viskupicova,J.,Vraka,P.S.,Zarkovi,N.,2010.
Naturalandsyntheticantioxidants:anupdatedoverview.FreeRadic.Res.44, 1216–1262.
Bisht, S., Feldmann, G., Soni, S., Ravi, R., Karikar, C., Maitra, A., Maitral, A., 2007. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novelstrategyforhumancancertherapy.J. Nanobiotechnol.,http://dx.doi. org/10.1186/1477-3155-5-3.
Box,G.E.P.,Hunter,W.G.,Hunter,J.S.,1978.StatisticsforExperimenters.JohnWiley &Sons,NewYork.
Cevc,G.,Vieri,U.,2010.Nanotechnologyandthetransdermalroute.Astateofthe artreviewandcriticalappraisal.J.Control.Release141,277–299.
Contri,R.V.,Fiel,L.A.,Pohlmann,A.R.,Guterres,S.S.,Beck,R.C.R.,2011.Transportof substancesandnanoparticlesacrosstheskinandinvitromodelstoevaluate skinpermeationand/orpenetration.In:Beck,R.C.R.,Guterres,S.S.,Pohlmann, A.R.(Eds.),NanocosmeticsandNanomedicine.,pp.3–35.
Das, R.K., Kasoju, N., Bora, U., 2010. Encapsulation of curcumin in alginate–chitosan–pluronic compositenanoparticles for delivery to cancer cells.Nanomed.Nanotechnol.6,153–160.
Farmacopeia Brasileira, 2010. Ministério da Saúde. Agência Nacional de Vig-ilância Sanitária, Brasília http://www.anvisa.gov.br/hotsite/cdfarmacopeia/ pdf/volume1%2020110216.pdf(accessedSeptember2011).
Gao,Y.,Li,Z.,Sun,M.,Guo,C.,Yu,A.,Xi,Y.,Cui,J.,Lou,H.,Zhai,G.,2011. Prepara-tionandcharacterizationofintravenouslyinjectablecurcuminnanosuspension. DrugDeliv.18,131–142.
Goel,A.,Kunnumakkara,A.B.,Aggarwal,B.B.,2008.Curcuminas“Curecumin”:from kitchentoclinic.Biochem.Pharmacol.75,787–809.
Guterres, S.S., Alves, M.P., Pohlmann, A.R., 2007. Polymeric nanoparticles, nanospheresandnanocapsulesforcutaneousapplications.DrugTargetInsights 2,147–157.
Irving,G.R.,Karmokar,A.,Berry,D.P.,Brown,K.,Steward,W.P.,2011.Curcumin:the potentialforefficacyingastrointestinaldiseases.BestPract.Res.Clin. Gastroen-terol.25,519–534.
Jayaprakasha,G.K., Rao,L.J.,Sakariah,K.K.,2006. Antioxidantactivitiesof cur-cumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 98, 720–724.
Jebors,S.,Leydier,A.,Wu,Q.,Ghera,B.B.,Malbouyre,M.,Coleman,A.W.,2010.Solid lipidnanoparticles(SLNs)derivedfrompara-acyl-calix[9]-arene:preparation andstability.J.Microencapsul.27,561–571.
Jenning,V.,Gysler,A.,Schafer-Korting,M.,Gohla,S.H.,2000.VitaminAloadedsolid lipidnanoparticlesfortopicaluse:occlusivepropertiesanddrugtargetingto theupperskin.Eur.J.Pharm.Biopharm.49,211–218.
Küchler,S.,Abdel-Mottaleb,M.,Lamprecht,A.,Radowski,M.R.,Haag,R., Schäfer-Korting,M.,2009.Influenceofnanocarriertypeandsizeonskindeliveryof hydrophilicagents.Int.J.Pharm.377,169–172.
Luengo,J.,Weiss,B.,Schneider,M.,Ehlers,A.,Stracke,F.,König,K.,Kostka, K.-H.,Lehr,C.M.,Schaefer,U.F.,2006.Influenceofnanoencapsulationonhuman skintransportof flufenamicacid. SkinPharmacol. Appl.SkinPhysiol. 19, 190–197.
Lyra,M.A.M.,Soares-Sobrinho,J.L.,Brasileiro,M.T.,Roca,M.F.L.,Barraza,J.A.,Viana, O.S.,Rolim-Neto,P.J.,2007.Sistemasmatriciaishidrofílicosemucoadesivospara liberac¸ãocontroladadefármacosLat.Am.J.Pharm.26,784–793.
Martin,A.N.,1993.PhysicalPharmacy:PhysicalChemicalPrinciplesinthe Pharma-ceuticalSciences,2nded.LippincottWilliams&Wilkins,Philadelphia,PA,USA, pp.622.
Martins,M.R.F.M.,Veiga,F.,2002.Promotoresdepermeac¸ãoparaaliberac¸ão trans-dérmicadefármacos:umanovaaplicac¸ãoparaasciclodextrinas.Rev.Bras. Farmacol.38,33–54.
Martins,R.M.,2014.Influênciademicroenanopartículaslipídicassólidasna eficiên-ciadeformulac¸õesfotoprotetorasbioativas.TesedeDoutorado,Faculdadede CiênciasFarmacêuticasdeRibeirãoPretodaUniversidadedeSãoPaulo,Ribeirão Preto,pp.181.
Mehnert,W.,Mader,K.,2001.Solidlipidnanoparticles:production,characterization andapplications.Adv.DrugDeliv.Rev.47,165–196.
Mourtas,S.,Canovi,M.,Zona,C.,Aurilia,D.,Niarakis,A.,LaFerla,B.,Salmona,M., Nico-tra,F.,Gobbi,M.,Antimisiaris,S.G.,2011.Curcumin-decoratednanoliposomes withveryhighaffinityforamyloid-1-42peptide.Biomaterials32,1635–1645.
Nair,H.B.,Sung,B.,Yadav,V.R.,Kannappan,R.,Chaturvedi,M.M.,Aggarwal,B.B., 2010.Deliveryofantiinflammatorynutraceuticalsbynanoparticlesforthe pre-ventionandtreatmentofcancer.Biochem.Pharmacol.80,1833–1843.
Nayak,A.P.,Tiyaboonchai,W.,Patankar,S.,Madhusudhan,B.,Souto,E.B.,2010.
Curcuminoida-loadedlipidnanoparticles:novel approachtowards malaria treatment.ColloidSurf.B81,263–273.
Oliveira,R.C.S.,2008.Desenvolvimento,formulac¸ãoeavaliac¸ãodesistemasde libertac¸ãotransdérmicaincorporandosistemasternáriosdecomplexac¸ão(Tese deDoutorado).UniversidadedoPorto,Portugal.
Paulucci,V.P.,Couto,R.O.,Teixeira,C.C.C.,Freitas,L.A.P.,2013.Optimizationofthe extractionofcurcuminfromCurcumalongarhizomes.Rev.Bras.Farmacogn.23, 94–100.
Puglia,C.,Frasca,G.,Musumeci,T.,Rizza,L.,Puglisi,G.,Bonina,F.,Chiechio,S., 2012.CurcuminloadedNLCinduceshistonehypoacetylationintheCNSafter intraperitonealadministrationinmice.Eur.J.Pharm.Biopharm.81,288–293.
Rusig,O.,Martins,M.C.,1992.Efeitodatemperatura,dopHedaluzsobreextratos deoleorresinadecúrcuma(CurcumalongaL.)ecurcumina.Rev.Bras.Cor.Nat. 1,158–164.
Sartorelli,P.,Anderson,H.R.,Angerer,J.,Corish,J.,Drexler,H.,Goen,T.,2000. Percu-taneouspenetrationstudiesforriskassessment.Environ.Toxicol.Pharmacol.8, 133–152.
Serra,M.L.G.,Vásquez,M.L.R.,Villafuerte,L.R.,Garcia,B.F.,Hernandez,A.L.,2009.
Efecto de los componentes de la formulación en las propiedades de las nanopartículaslipídicassólidas.Rev.Mex.Cienc.Farm.40,26–40.
Suwantong,O.,Waleetorncheepsawat,S.,Sanchavanakit,N.,Pavasant,P., Cheep-sunthorn, P., Bunaprasert,T., Supaphol, P., 2007. Invitro biocompatibility of electrospun poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate)fibermats.Int.J.Biol.Macromol.40,217–223.
Tønnesen,H.H.,Karlsen,J.,1985.Studiesoncurcuminandcurcuminoids:VI– kinet-icsofcurcumindegradationinaqueoussolution.Z.Lebensm.Unters.For.180, 402–404.
Yallapu,M.M.,Jaggi,M.,Chauhan,S.C.,2012.Curcuminnanoformulations:afuture nanomedicineforcancer.DrugDiscov.Today17,71–80.
Yallapu,M.M.,Jaggi,M.,Chauhan,S.C.,2013.Curcuminnanomedicine:aroadto cancertherapeutics.Curr.Pharm.Design19,1994–2010.
Wissing,S.A.,Lippacher,A.,Muller,R.H.,2001.Investigationsontheocclusive prop-ertiesofsolidlipidnanoparticles(SLN).J.Cosmet.Sci.52,313–324.
Zamarioli,C.M.,2014.Formulac¸ãotópicaparaprevenc¸ãoetratamentode radio-dermites:desenvolvimentodenanopartículaslipídicassólidas(NLS)contendo curcuminoideseestudoinvitro.Dissertac¸ãodeMestrado,EscoladeEnfermagem deRibeirãoPretodaUniversidadedeSãoPaulo,RibeirãoPreto,pp.139.
ZetasizerNano-ZS,2009.UserInstructions.NBTCUserInstructions.