www.rpped.com.br
REVISTA
PAULISTA
DE
PEDIATRIA
ORIGINAL
ARTICLE
Frequency
of
prescriptions
of
off-label
drugs
and
drugs
not
approved
for
pediatric
use
in
primary
health
care
in
a
southern
municipality
of
Brazil
Marcele
Giacomin
Gonc
¸alves
∗,
Isabela
Heineck
FaculdadedeFarmácia,UniversidadeFederaldoRioGrandedoSul(UFRGS),PortoAlegre,RS,Brazil
Received3March2015;accepted16June2015 Availableonline30December2015
KEYWORDS Pediatrics; Medicationuse; Primaryhealthcare
Abstract
Objective: To determine the frequency of prescriptions of off-label drugs and drugs not approvedforpediatricuseinprimaryhealthcareinmedium-sizedmunicipalityofRioGrande doSul,Brazil.
Methods: Cross-sectional studywith retrospectivedatacollection, whichanalyzed prescrip-tionsissuedto326patientsfromAugusttoDecember/2012intwobasichealthunitsinthecity ofViamão,stateofRioGrandedoSul.Itincludedallprescriptionsofpatientswhosemedical recordsorservicerecordswereavailable andcomplete inrelationtothedateofpresence, weight anddate ofbirth.Off-label prescriptionswerethose which,inrelationto thedrug leaflet,showeddosedifferenttherecommendedrange,frequencyofprescriptionand/or dif-ferentformofadministrationandyoungeragethantheindicatedrange.Descriptivestatistics withabsolutefrequencies,meansandstandarddeviationswereused.
Results: Duringthestudyperiod,atotalof731drugprescriptionswereissuedandthefrequency ofoff-labelmedicationsprescribed was31.7%,especially antihistaminesandantiasthmatics (32.3% and31.5%, respectively). The maintype of off-labelprescription wasdose (38.8%), followedbyagerange(31.5%)andfrequencyofadministration(29.3%).Regardingthedose off-labelprescription,overdosewasmorefrequent(93.3%)thantheunderdose(6.7%).Prescriptions ofunapproveddrugswerenotidentified.
Conclusions: Thestudyshowed thatofflabelprescriptioniscommoninbothassessedunits. TheobservedpercentageofofflabelprescriptionwashigherthanthatreportedbyEuropean studiescarriedoutinprimarycare.Ontheotherhand,theprescriptionofdrugsnotapproved forchildrenwasnotobserved.
©2015SociedadedePediatriadeS˜aoPaulo.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBYlicense(https://creativecommons.org/licenses/by/4.0/).
∗Correspondingauthor.
E-mail:marcelegg@yahoo.com.br(M.G.Gonc¸alves).
http://dx.doi.org/10.1016/j.rppede.2015.06.023
PALAVRAS-CHAVE Pediatria;
Usode
medicamentos; Atenc¸ãoprimária àsaúde
Frequênciadeprescric¸õesdemedicamentosofflabelenãolicenciados parapediatrianaatenc¸ãoprimáriaàsaúdeemmunicípiodosuldoBrasil
Resumo
Objetivo: Determinarafrequênciadeprescric¸õesdemedicamentosofflabelenãolicenciados parapediatrianaatenc¸ãoprimáriaàsaúdeemmunicípiodemédioportedoRioGrandedoSul, Brasil.
Métodos: Estudo transversal, com coleta retrospectiva, que analisou prescric¸ões a 326 pacientesemitidasdeagostoadezembro de2012em doispostosdesaúdedomunicípiode Viamão.Foramincluídastodasasreceitasdepacientescujosprontuáriosoufichasde atendi-mentoestivessemdisponíveisecompletosemrelac¸ãoàdatadeatendimento,pesoedatade nascimento.Foramclassificadascomo prescric¸ões offlabelaquelas que,emrelac¸ão àbula do medicamento, apresentavam dose diferente da recomendada,frequência de prescric¸ão e/ouformadeadministrac¸ãodiferenteeidadeinferioràquelaindicada.Foiusadaestatística descritiva,comfrequênciasabsolutas,médiasedesviopadrão.
Resultados: Durante operíodo estudadohouvea prescric¸ão de731 medicamentosehouve frequênciade31,7%demedicamentosprescritosofflabel,especialmenteanti-histamínicose antiasmáticos(32,3%e31,5%,respectivamente).Oprincipaltipodeprescric¸ãoofflabelfoi dose(38,8%),seguidadeidade(31,5%)edefrequênciadeadministrac¸ão(29,3%).Comrelac¸ão àprescric¸ãoofflabeldedose,foimaisfrequenteasobredose(93,3%)doqueasubdose(6,7%). Nãoforamencontradasprescric¸õesdemedicamentosnãolicenciados.
Conclusões: Oestudomostrouqueaprescric¸ãoofflabelécomumnasduasunidadesestudadas. Opercentualdeprescric¸ãoofflabelobservadofoisuperioraorelatadoporestudoseuropeus feitosnaatenc¸ãoprimária.Poroutrolado,nãofoiobservadaprescric¸ãodemedicamentosnão licenciadosparacrianc¸as.
©2015SociedadedePediatriadeS˜aoPaulo.PublicadoporElsevierEditoraLtda.Esteéumartigo OpenAccesssobalicençaCCBY(https://creativecommons.org/licenses/by/4.0/deed.pt).
Introduction
Considering the lack of drugs for use in children, espe-ciallythoseunder2yearsofage,theoff-labelprescription drugshavebecomearoutinepracticebothinhospitalsand ambulatoryandbringsdoubtstoprescribersandproviders regardingthebenefittothepediatricpatient.1,2
Theterm‘‘off-label’’referstodrugsprescribedin differ-entmannerthanthatdirectedintheinstructionsorofficial compendiainrelationtodose,indication,agegroup,dosing interval,or formofadministration.3Off-labelprescription
isnotillegal;itis notnecessarilyincorrectandis present in several pediatric protocols. The quality of drug ther-apyisnotnecessarilyrelatedtothelicensingstatusofthe drug.However,thereareseveralclinical,ethical,andsafety factorsthatshouldbeconsideredandthereareno guide-lines toassist off-label prescription. The decision onthis typeofprescriptionshouldbeassessed accordingto clini-calindication,treatmentoptions,andrisk-benefitanalysis. Moreover, it must obtain the patient’s or guardian con-sent,takingcaretoavoidexposingchildrentounnecessary risks.4
Regarding the concept of unlicensed medicine, some authorsconsiderthatitreferstodrugsthatareunregistered inthe surveillance agency,or are extemporaneous prepa-rations,or drugscontainingnon-pharmacologicalchemical ingredientsusedwiththerapeuticpurpose.5---8Ferreiraetal.
extendthe conceptofunlicensedtoregistered drugsthat arecontraindicatedforchildren.9
There arestudies that characterize the extent of off-labelprescribinginpediatrichospitalsinBrazil,9---11butlittle
is knownof outpatientprescription in primary care. This studyaimstofillthisgap,determinethefrequencyof pre-scriptionofoff-labelandunlicenseddrugsforpediatrics,to supportthedevelopmentofactionstopromoterationaluse ofdrugs.
Method
Cross-sectional study with retrospective data collection, approved by the Research Ethics Committee of the Uni-versidadeFederal doRioGrandedoSul(No.214535)and authorizedbytheMunicipalSecretaryofHealthofViamão. Data collection wasperformed in two basic health units: FamilyHealthStrategy(FHS)ItapuãandReferenceUnit(RU) LombaSabão,Viamão.Viamãoisacityofthemetropolitan arealocated20.6kmfromthecapitalPortoAlegre,withan areaof 1494.26km2and 239,384inhabitants, accordingto
theDemographicCensusoftheBrazilianInstituteof Geog-raphyandStatistics(IBGE)2010.12
Samplesizecalculationwasperformedconsidering3759 pediatric consultations in health facilities involved dur-ing a period of five months (August---December 2012). A 20%expectedfrequencyofoff-labelprescriptiondrugswas used.4---8,13---16 Toa95%confidenceinterval,a20%+5%range
Copies of prescriptions retained in the unit dispen-sary after dispensing were evaluated, withmedication of patientsunder theageof 12 attendedbypediatriciansof therespectivehealthunits,fromAugusttoDecember2012. We excluded prescriptions for patients not linked to the unit.Unituserprescriptionswhosemedicalrecordswerenot foundorwereincompleteregardingthevariablesofinterest werenotconsidered.
Prescriptiondatawererecordedonaspecifictableand supplemented with information from medical records or patient’s chart. The variables of interest related to the patient (age, sex, and weight) and prescriptions (total items,prescriptiondrugs,presentations,dosageform,route of administration, frequency of administration, anddose) wererecorded.
Fordataanalysispatientsweredividedintofourgroups according to age: infants (0---2 years), preschoolers(>2---7 years), schoolers (>7---10 years), and adolescents (>10---12 years). The prescribed drugs were classified according to ANVISAElectronic Labeling(Bulário Eletrônico da Agência Nacional deVigilância Sanitária)17 and, in the absenceof
informationonthissite,instructionsprovidedbythe manu-facturerintothreeclassifications:accordingtospecification (age,dose,frequencyofadministrationandformof admin-istration,asspecifiedinthepackageinsert);off-label(drugs prescribedfordifferentages,athigherorlowerdoses,with differentdosingfrequencyandadministrationmannerother thantheindicated)18;andnotlicensed forchildren(drugs
forwhichtherewasnoinformationorwerecontraindicated forchildren,9registrationandlabelingonlyconsideredthe
adultuse).
The Anatomical Therapeutic Chemical Classification (ATC)19 wasusedtoenabletheanalysisofdrugsby
thera-peuticclasses.DatawereorganizedinMicrosoftOfficeExcel 2007spreadsheetandanalyzedusingtheSPSS18.0software. Descriptivestatisticswithabsolute frequencies(meanand standarddeviation)wasused.
Results
Prescriptionsfor705pediatricpatientswereretainedinthe studyperiodinselectedunits,generallymorethanone pre-scriptionperpatient.Only326oftheseprescriptionswere includedinthestudy,as379recordswerenotfoundorwere incomplete.Regardingcareunits,203(62.3%)patientswere fromtheFamilyHealthStrategy(FHS)Itapuãandtheother 123 (37.7%) from the Reference Unit (RU) Lomba Sabão. Ofthe326children,56.4%weremale. Therewasahigher prevalenceofinfants, 142(43.3%);followedby preschool-ers,103 (31.6%).Schoolers 55(16.9%) andadolescents 27 (8.3%)wereminorityandamountedto25.2%ofthepatients. Amonginfants(0---2years),89(63%)wereupto12months oldand53(37%)from13monthsto2yearsold.The num-ber of drugs ranged from one to eight, with an average of 2.2±1.4 per patient.According to the analysisof pre-scriptions, 95.4% of all prescribed drugs belonged to the municipallistofessentialmedicines(RESUME).
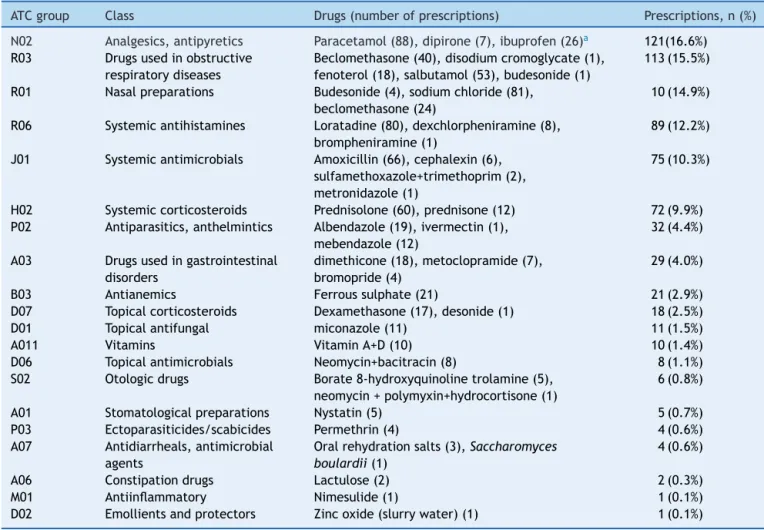
Intotal,39differentactiveprinciples(30isolatesand9 combinations) wereprescribedin different presentations. The most frequently prescribed drugs were: paracetamol 88(11.8%);nasal saline81(11.1%);loratadine80 (10.3%);
amoxicillin66(8.3%);prednisolone60(8.2%),andoralspray salbuterol53(7.3%).Table1describesthefrequencyof pre-scriptionbytherapeuticclass.
Wefound731 prescribeddrugs.Therewasno prescrip-tionofunlicensed drugsfor children. Allprescribeddrugs wereforadultandpediatricuse.Ofthetotal,232(31.7%) prescriptionswereoff-labeland13(0.02%)hadno specifi-cationoftheprescribeddose,onlythenameoftheactive ingredient.Drugsnotspecifyingthedosewere:vitaminA+D andpermethrin(for9[1.2%]and4[0.6%]children, respec-tively).Theclassificationofprescribeddrugsissummarized inTable2.
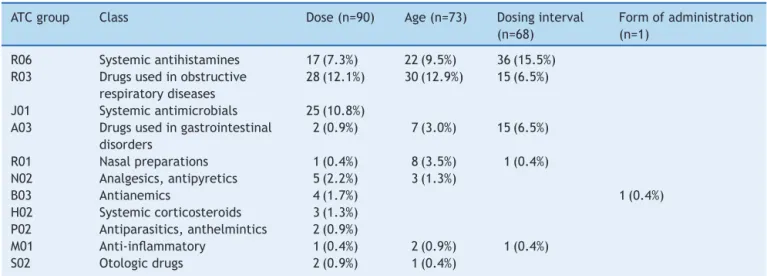
Among the off-label prescriptions, the following types andfrequencieswereseen:off-labeldose,90(38.8%); fol-lowedbyage,73(31.5%);andfrequencyofadministration, 68(29.3%).Regardingoff-labeldose,overdosing wasmore prevalentthanunderdosing:84(93.3%)vs.6(6.7%) prescrip-tions,respectively.
Theoff-labelprescribeddrugsarepresentedinTable3by therapeuticclasses.Amongthem,wehighlightloratadine, oralspraysalbutamol,fenoterol,anddimethicone.
Loratadinewasthethird mostprescribeddrugandhad afrequency ofoff-labelprescription of 85.3%,dosing fre-quency of 53.1%, younger age than the recommended of 25%,and overdosing of 21.9%.Regarding prescriptions for off-labelfrequencyofadministration,19(55.9%)werealso off-label dose; that is, two types of discordant recom-mendeduse.
Salbutamol, whose frequency of prescription was 7.3% (53)wasprescribedoff-labelin100%ofprescriptions: indi-catedfor an agegroupyoungerthantherecommendedin 27(50.9%)andforuseindoseshigherthanrecommendedin thepackageinsertin26(49.1%)cases.
Fenoterolhad18prescriptions.Onlyoneofthemwasin accordancewiththepackage insert, the other 17(94.4%) were classified as off-label. Of these, 15 (88.2%) were off-label for frequency of administration above the rec-ommendedand two(11.8%) due to the higher dose than recommended.Thetwoprescriptionswithdosehigherthan recommendedalsohad frequencyof administrationabove therecommended.
Dimethicone,amedication out ofthe municipallistof essentialmedicines,wasprescribedin 17occasions,16of them(94.1%)off-label:14forfrequencyof administration abovetherecommendedandtwiceforoverdosing.Inboth casesofoverdosingtherewasalsotheincorrectprescribing offrequencyofadministration.
Intheanalysisofoff-labelprescribeddrugsbyagegroup, it is noteworthily the prescription of loratadine and dex-chlorpheniramineforinfants----anagegroupforwhichthese drugsarenotrecommended.Inthegroupofpreschoolers, itisworthnotingtheprescriptionofsalbutamoland amoxi-cillinatdoseshigherthanrecommended.Intheagegroups correspondingtoschoolersandadolescents,theoverdoseof salbutamolwaswhatstoodout.
Discussion
Therewasnoprescriptionofunlicenseddrugs.Otherauthors foundpercentages,whichvariedfrom0.3to16.8%.5,14,18The
Table1 Therapeuticclassofprescribeddrugsandprescriptionfrequency.
ATCgroup Class Drugs(numberofprescriptions) Prescriptions,n(%)
N02 Analgesics,antipyretics Paracetamol(88),dipirone(7),ibuprofen(26)a 121(16.6%)
R03 Drugsusedinobstructive
respiratorydiseases
Beclomethasone(40),disodiumcromoglycate(1), fenoterol(18),salbutamol(53),budesonide(1)
113(15.5%)
R01 Nasalpreparations Budesonide(4),sodiumchloride(81),
beclomethasone(24)
10(14.9%)
R06 Systemicantihistamines Loratadine(80),dexchlorpheniramine(8),
brompheniramine(1)
89(12.2%)
J01 Systemicantimicrobials Amoxicillin(66),cephalexin(6),
sulfamethoxazole+trimethoprim(2), metronidazole(1)
75(10.3%)
H02 Systemiccorticosteroids Prednisolone(60),prednisone(12) 72(9.9%)
P02 Antiparasitics,anthelmintics Albendazole(19),ivermectin(1),
mebendazole(12)
32(4.4%)
A03 Drugsusedingastrointestinal
disorders
dimethicone(18),metoclopramide(7), bromopride(4)
29(4.0%)
B03 Antianemics Ferroussulphate(21) 21(2.9%)
D07 Topicalcorticosteroids Dexamethasone(17),desonide(1) 18(2.5%)
D01 Topicalantifungal miconazole(11) 11(1.5%)
A011 Vitamins VitaminA+D(10) 10(1.4%)
D06 Topicalantimicrobials Neomycin+bacitracin(8) 8(1.1%)
S02 Otologicdrugs Borate8-hydroxyquinolinetrolamine(5),
neomycin+polymyxin+hydrocortisone(1)
6(0.8%)
A01 Stomatologicalpreparations Nystatin(5) 5(0.7%)
P03 Ectoparasiticides/scabicides Permethrin(4) 4(0.6%)
A07 Antidiarrheals,antimicrobial
agents
Oralrehydrationsalts(3),Saccharomyces boulardii(1)
4(0.6%)
A06 Constipationdrugs Lactulose(2) 2(0.3%)
M01 Antiinflammatory Nimesulide(1) 1(0.1%)
D02 Emollientsandprotectors Zincoxide(slurrywater)(1) 1(0.1%)
ATC,anatomicaltherapeuticchemicalclassification.
aIbuprofenprescribedasananalgesicandantipyretic.
tothehighadherence(95.4%)ofViamãopediatricianstothe listofessentialmedicinesinthemunicipality (RESUME).A lowerpercentageofadherencetotheRESUME(76.4%)was seeninastudyperformedineightcitiesofthreeBrazilian states.20Theuseoflistsofessentialmedicinesisameasure
recommendedbytheWorldHealthOrganizationtopromote therationaluseofdrugs.21Theavailabilityofasmaller
ther-apeuticarsenalandthattakesintoaccountthehealthneeds ofthemajorityofthepopulationcanreducethechanceof usingunlicensedproducts.
On the other hand, the prescription of off-label drugs in primary care in Viamão was high, with a frequency
Table 2 Classification of prescribed drugs regarding its packageinsertspecification.
Classification Frequency,n(%)
Accordingtospecification 486(66.5%)
Unlicensed 0
Off-labelprescribing 232(31.7%) Unabletoclassifya 13(1.8%)
Totaldrugsprescribed 731(100%)
aUnabletoclassifybecausetherewasnopharmaceuticalform
onprescription.
of 31.7% above the range found in other European population-basedstudies:Scotland(24.6%),England(16%), Netherlands(20.3%),Estonia(31%),Italy(17%),andFrance (29%).6,8,13,15,22,23Themaintypesofoff-labelprescriptionin
this study weredose (38.8%),age (31.5%), andfrequency of administration (29.3%). Similarly, the above-mentioned Europeanstudiesindicateddoseandageasthemaintypes ofoff-labeluse,inthatorder.Thefrequencyofdrug admin-istrationwasnotamongtheoff-labeltypes,whichprobably is due tothe fact that other authors evaluatedthe dose andfrequencyofadministrationtogetherandclassifiedthe casesoutofspecificationasoff-labeldose.
Overdosingwasmorefrequent,unlikereportedbyother authorswhofound a greaternumberof underdosing, par-ticularly for the class of antimicrobial drugs for systemic use.13,14,24 There was aprevalence of 10.3% of
antimicro-bialprescriptions,whichrepresented10.8%oftotaloff-label prescriptions, mostly by overdosing. This result shows a differenttrendfromthatofotherstudiesassociating under-dosing withphysiciansdifficulty toadjust thedose tothe child’s age, that is, to know the age and certain situa-tionsinwhichdosesshouldbeincreased.24Amoxicillin,the
mostprescribedantimicrobialinthisstudy,hasadose indi-cation on label less than that of other sources, such as theFormulárioTerapêuticoNacional.25 The datasuggest a
Table3 Typeofoff-labelusebytherapeuticclass.
ATCgroup Class Dose(n=90) Age(n=73) Dosinginterval
(n=68)
Formofadministration (n=1)
R06 Systemicantihistamines 17(7.3%) 22(9.5%) 36(15.5%) R03 Drugsusedinobstructive
respiratorydiseases
28(12.1%) 30(12.9%) 15(6.5%)
J01 Systemicantimicrobials 25(10.8%) A03 Drugsusedingastrointestinal
disorders
2(0.9%) 7(3.0%) 15(6.5%)
R01 Nasalpreparations 1(0.4%) 8(3.5%) 1(0.4%)
N02 Analgesics,antipyretics 5(2.2%) 3(1.3%)
B03 Antianemics 4(1.7%) 1(0.4%)
H02 Systemiccorticosteroids 3(1.3%) P02 Antiparasitics,anthelmintics 2(0.9%)
M01 Anti-inflammatory 1(0.4%) 2(0.9%) 1(0.4%)
S02 Otologicdrugs 2(0.9%) 1(0.4%)
Percentageoftotaloff-labelprescribing(232);ATC,anatomicaltherapeuticchemicalclassification.
Electronic Labeling and reinforce the need to constantly update this source of information on the part of manu-facturers and the product license review by the agency. Anotherimportantmeasurewouldbetoestablisha munic-ipal Pharmacyand Therapeutics Committeeaiming at the developmentof protocolsfor theuse of themedicines in the list according to recent studies, to support the pre-scriber and promote the rational use of medicines. It is worthrememberingthatthequalityofdrugtherapyisnot necessarilyrelatedtothedrug’sregulatorystatus.
The most commonly prescribed off-label drugs were the anti-asthmatics and antihistamines for systemic use (Table 3). These classes of drugs are among the most commonly prescribedin pediatric primarycare.6,16 In this
study,loratadinewasprescribedoff-labelin85.3%ofcases regardingfrequency ofadministration(53.1%),lower than recommended age group (25%), and overdosing (21.9%). Considering that the drug’s plasma half-life is 17---24h,25
there would be no need or indication for multiple daily doses,asobservedinthisstudy.Theuseofantihistamines in a way not appropriate for the age has been reported in recent literature review that showed a variation of 6.5---43%.26 Da Costa et al. reported Loratadineas oneof
thedrugshardtodealwithinpediatricsduetothe restric-tionforageunder2years.1TheusualofLoratadineis5mg
forchildren2---6yearsoldandunder30kgand10mg once-dailyforchildrenover6yearsoldandadults.25,26Thestudy
showedoverdosing inprescriptionsofloratadine.Onlyone studyadvocatedtheuseofahigherdosethanthatrequired inthepackageinsertfortreatingallergicasthma.27
Consid-ering that such information is not confirmed in the 2012 GuidelinesoftheBrazilianSocietyofPneumologyand Phthi-siologyfortheManagementofAsthma(SociedadeBrasileira dePneumologiaeTisiologiaparaoManejodaAsma),itis evi-denttheneedforcontinuingeducationofprofessionalsand dose standardization basedonclinical trials and,ifnone, observationalstudies.28
Therewasaprevalenceofoff-labelprescribingof31.5% for respiratory diseasemanagement (Table 3), salbutamol sprayand fenoterolnebulizer solutionstanded outamong themedications.Treatment of asthma inchildren is chal-lengingand oftenan overlapbetweenrecurrentwheezing
andasthmaphenotypesoccurs,makingdiagnosticand ther-apeuticdecisionscontroversial.28 Recommendationsofthe
BrazilianSocietyofPneumologyandPhthisiology(SBPT) dif-ferfromthepackageinsertrecommendationsforsalbutamol spray.Theguidelinesindicatetheuseinchildrenover5years oldandwithhigherdosesthanthoseindicatedbythe man-ufacturer,evenwithpossiblelimitationstothecorrectuse of the device in this age group. The drug package insert highlightsthedifficultyofthedevicecorrectuseinchildren under7years,withnorestrictions for useabove thisage group.Consideringthelargenumberofhospitalizationsand therisksofnon-treatmentofanasthmaattack(suffocation istheleadingcauseofdeathinalmostallcases),28
adapt-ingtheadultdoseforchildrenisarecurringpracticebased onclinician’sknowledge,butwithlittledocumentationby Brazilianphysicians.25Althoughtheclinicalbenefitofthese
drugs in acute asthma management is well documented, thereisgreatvariabilityinthedosesused,mostlybasedon expertopinion,clinicalconsensus,orstudieswithalimited numberof patients. Littleevidence precisely supportthe dosestobeused.28
Theuseofsalbutamol,abeta-adrenergicagonist,inhigh dosesis associatedwith tremors, agitation,hypokalemia, andcardiacarrhythmias.Thereisevidencethattreatment withsalbutamolshiftsthecardiovascularautonomic regula-tiontoanewlevel,characterizedbyagreatersympathetic response and mild 2-receptor tolerance. Based on this evidence,salbutamol abusemay be a substratefor atrial fibrillation.29
The mostimportantdeterminantof thedailydosage is clinical judgmentof the patient’s responseto treatment. Thedoctorshouldmonitorthepatient’sresponseandadjust the dose according to the level of asthma control. Low, mediumorhighdosessettingsarebasedonmanufacturers’ pharmacokineticandpharmacodynamicstudies,which are rarelybasedondose-responsecurves.Theyvaryaccording tothedeviceusedandmustbeassessedindividually.28
Mostmedicines used bychildren are prescribedin pri-mary care, and pediatricians or general practitioners use arelativelysmallnumberof drugstosolvethemost com-mon problems. The small cast of medicines facilitates the development of municipal clinical protocol, built in amultidisciplinary wayin thePharmacy andTherapeutics Committee.Thiscontrastswithsecondarycareinwhichthe numberofchildrenislowercomparedtothegeneral popu-lation,butamuchlargernumberofdrugsisprescribedto treatrareandmoreseriousconditions.13
Extrapolationofthisstudy’sresultsshouldbedonewith caution, as data refer to two health units, a five-month period,andhalfoftheprescriptionscouldnotbeassessed duetolackofinformation. Prescribinghabitsvary accord-ing to the formation of pediatricians, protocols, or even serviceroutines.Studiesinvolvingalargernumberofhealth units areneeded toestablish more accurately the extent ofoff-labelprescribinginmunicipalorregionallevel. Fur-thermore,thelimitationsinherentinretrospectivestudies shouldbeconsidered.Informationwascollectedfrom pre-scriptionsretainedinpharmaciesandmanualrecords.The incompleterecordof thenameof patientsandthe differ-entcriteriaofcatalogingrecordsdeterminedtheexclusion ofsomecases.The retrospectivedatacollectionalso pre-vented the analysis of off-label usage by indication, as thisdatawasrarely foundin medicalrecordsor patients’ charts. We should also consider that only the prescrip-tions dispensed are retained in the pharmacies of these units.Patientsmayhavereceivedotherprescriptionofdrugs subjecttospecialcontrol,notpresentinREMUME,orof spe-cialorspecializedcomponentofthepharmaceuticalcare. Despitetheselimitations,thisstudybringscontributions,as the Brazilian data on the use of unlicensed and off-label medicinesinchildrenaresofarrestrictedtohospitals.
Thedifficultiesrelatedtoresearchwithchildrenfoster off-labelprescribing.Althoughthispracticeisnotillegal,it createsuncertainty regardingthepossible adverseeffects in a population with specific characteristics such as the pediatric population. The present study showed that this practiceiscommoninprimaryhealthcare inacityofRio GrandedoSul,similartostudiesinEuropeancities.Wehope thattheresultsmaycontributetotheplanningofactionsto supportprescribersandprovidegreatersecurityintheuse ofmedicinesforpediatricpatients.
Funding
Thestudyreceivednofunding.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.Da Costa PQ, Rey LC, Coelho HL. Carência de preparac¸ões medicamentosaspara o usoem crianc¸as noBrasil. JPediatr (RioJ).2009;85:229---35.
2.FuchsFD,WanmacherL.Farmacologiaclínica:fundamentosda terapêuticaracional.4a
ed.RiodeJaneiro:GuanabaraKoogan; 2010.
3.Brasil---MinistériodaSaúde.Anexo03:Protocolodeseguranc¸a naprescric¸ão,usoeadministrac¸ãodemedicamentos[página na Internet]. Retrieved from: http://www.pa2online.org/ abstracts/Vol7Issue4abst143P.pdf.
4.LangerováP,VrtalJ,UrbánekK. Incidenceofunlicensedand off-labelprescriptioninchildren.ItalJPediatr.2014;40:12. 5.McIntyreJ,ConroyS,AveryA,CornsH,ChoonaraI.Unlicensed
andofflabelprescribingofdrugsingeneralpractice.ArchDis Child.2000;83:498---501.
6.PandolfiniC,CampiR,ClavennaA,CazzatoT,BonatiM.Italian paediatriciansandoff-labelprescriptions:loyaltoregulatoryor guidelinestandards.ActaPaediatr.2005;94:753---7.
7.MühlbauerB,JanhsenK,PichlerJ,SchoettlerP.Off-labeluseof prescriptiondrugsinchildhoodandadolescence.DtschArztebl Int.2009;106:25---31.
8.LassJ,IrsA,PisarevH,LeinemannT,LutsarI.Offlabeluseof prescriptionmedicinesinchildreninoutpatientsettingin Esto-niaiscommon.PharmacoepidemiolDrugSaf.2011;20:474---81. 9.FerreiraLA, IbiapinaCC, MachadoMG, FagundesED. A alta prevalência de prescric¸ões de medicamentos off label e não licenciados em unidade de terapia intensiva pediátrica brasileira.RevAssocMedBras.2012;58:82---7.
10.Dos Santos L, Heineck I. Drug utilization study in pediatric prescriptions ofa university hospital insouthern Brazil: off-label, unlicensed and high-alert medications. Farm Hosp. 2012;36:180---6.
11.SantosDB,ClavennaA,BonatiM,CoelhoHL.Off-labeland unli-censeddrug utilizationinhospitalizedchildren inFortaleza, Brazil.EurJClinPharmacol.2008;64:1111---8.
12.IBGE---InstitutoBrasileirodeGeografiaeEstatística,Cidades --- Rio Grande do Sul. Retrieved from: http://www.cidades. ibge.gov.br/xtras/perfil.php?lang=&codmun=432300&search= rio-grande-do-sul|viamao[accessed04.06.15].
13.Ekins-DaukesS,HelmsPJ,SimpsonCR,Taylor MW,MclayJS. Off-labelprescribingtochildreninprimarycare:retrospective observationalstudy.EurJClinPharmacol.2004;60:349---53. 14.’t Jong GW,Eland IA,Sturkenboom MC, vanden Anker JN,
StrickerfBH.Unlicensedandoff-labelprescriptionof respira-torydrugstochildren.EurRespirJ.2004;23:310---3.
15.OlssonJ,KimlandE,PetterssonS,OdlindV.Paediatricdruguse withfocusonoff-labelprescriptionsinSwedishoutpatientcare ---anationwidestudy.ActaPaediatr.2011;100:1272---5. 16.Brasil---MinistériodaSaúde.SecretariadeCiência,Tecnologia
eInsumosEstratégicos.Usoofflabel:erroounecessidade?Rev SaudePublica.2012;46:398---9.
17.ANVISA --- Agência Nacional de Vigilância Sanitária, Bulário Eletrônico. Retrieved from: http://www.anvisa.gov.br/ datavisa/filabula/index.asp[accessed04.06.15].
18.ChalumeauM,TreluyerJM,SalanaveB,AssathianyR,Chéron G, Crocheton N, et al. Off label and unlicensed drug use among French office based paediatricians. Arch Dis Child. 2000;83:502---5.
19.ATC --- Anatomical Therapeutic Chemical Classification. Retrieved from: http://www.whocc.no/atcdddindex/ [accessed04.06.15].
20.DalPizzolTS,TrevisolDJ,HeineckI,FloresLM,CamargoAL, KöenigA, etal. Adesãoa listasde medicamentosessenciais emmunicípiosdetrêsestadosbrasileiros.CadSaudePublica. 2010;26:827---36.
21.World Health Organization. Promoting rational use of medicines: core components. WHO Policy Perspectives on Medicine.Geneva:WHO;2002.
22.HelmsPJ,DaukesSE,TaylorMW,SimpsonCR,MclayJS.Utility ofroutinelyacquiredprimarycaredataforpaediatricdisease epidemiologyandpharmacoepidemiology.BrJClinPharmacol. 2005;59:684---90.
primary care.Proc Br PharmacolSoc. 2015. Retrieved from: http://www.pA2online.org/abstracts/Vol7Issue4abst143P.pdf [accessedFebruary2015].
24.Ekins-DaukesS,MclayJS,Taylor MW,SimpsonCR, HelmsPJ. Antibiotic prescribing for children. Too much and too little? Retrospective observationalstudy in primary care.Br JClin Pharmacol.2003;56:92---5.
25.Brasil---MinistériodaSaúde.SecretariadeCiência,Tecnologiae InsumosEstratégicos.DepartamentodeAssistência Farmacêu-ticaeInsumos Estratégicos. Formulário terapêutico nacional 2010--- Rename2010.2a
ed.Brasília:MinistériodaSaúde;2010. 26.SilvaD,AnsoteguiI,Morais-AlmeidaM.Off-labelprescribingfor allergicdiseasesinChildren.WorldAllergyOrganJ.2014;7:4.
27.MenardoJ-L,HorakF,DanzigMR,CzarlewskiW. Areviewof loratadineinthetreatmentofpatientswithallergicbronchial asthma.ClinTher.1997;19:1278---93.
28.SociedadeBrasileiradePneumologiaeTisiologia.Diretrizesda SociedadeBrasileiradePneumologiaeTisiologiaparaoManejo daAsma.JBrasPneumol.2012;38:S1---46.
29.Patanè S,MarteF,La RosaFC,La RoccaR.Atrialfibrillation associatedwithchocolateintakeabuseandchronicsalbutamol inhalationabuse.IntJCardiol.2010;145:74---6.