w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Clinical
safety
evaluation
of
a
tea
containing
Cissampelos
sympodialis
in
healthy
volunteers
Liane
Franco
Barros
Mangueira
a,∗,
Luciana
da
Silva
Nunes
Ramalho
a,
Andressa
Brito
Lira
a,
Josué
do
Amaral
Ramalho
a,
Kardilandia
Mendes
Oliveira
a,
Aretuza
Iolanda
Pimentel
de
Almeida
Torres
a,
Valério
Marcelo
Vasconcelos
do
Nascimento
b,
Caliandra
Maria
Bezerra
Luna
Lima
a,
Cícero
Flávio
Soares
Aragão
c,
Margareth
de
Fátima
Formiga
Melo
Diniz
aaLaboratóriodeAnálisesToxicológicas,CentrodeCiênciasdaSaúde,UniversidadeFederaldaParaíba,JoãoPessoa,PB,Brazil bInstitutodoCorac¸ãodaFaculdadedeMedicina,Servic¸odeEcocardiografia,UniversidadeFederaldeSãoPaulo,SãoPaulo,SP,Brazil cDepartamentodeFarmácia,CentrodeCiênciadaSaúde,UniversidadeFederaldoRioGrandedoNorte,Natal,RN,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received12January2015
Accepted19June2015
Availableonline29July2015
Keywords:
Clinical
Cissampelossympodialis
Menispermaceae Tea
a
b
s
t
r
a
c
t
CissampelossympodialisEichler,Menispermaceae,iswidelyusedbyIndiantribesandfolkmedicineto treatvariousinflammatorydisorders,includingasthma.Clinicaltoxicologicaltrialsweremadewiththe teaofC.sympodialis,amedicinalplant.ThestudytookplaceatLauroWanderleyHospital/UFPB-PB,where seventeenhealthyvolunteerswerechosen,amongthosesixmenandelevenwomenwhoorallyingested, duringfourweeksuninterruptedly,150mlofthetea,onceaday.Beforethefirstingestionandafterthe lastone,theparticipantsweresubjectedtoclinicalandlaboratorialtestsfortheiroverallconditionsin ordertoanalyzethetoxicityoftheplant.Theresultsdemonstratedthatthevolunteersneitherexperience clinicalnorlaboratorialalterations,aswellasnosignificantadverseeffects,apartfromlittlechange detectedintheirhematologicaltests.Nevertheless,nonedemonstratedanypathologicalconditions,just alterationsofthenormalhumanbeingphysiology.Therefore,itisconcludedthatthesedatacomplement thatobtainedduringpre-clinicalstudiesandconfirmalowtoxicityofthisplant.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Theherbalinfusionisadrinkmadefromleaves,flowers,seeds,
fruit,stalksandsomeplantspeciesroots(Zhaoetal.,2013;Anvisa,
2010).Thepreparationconsistsofpouringboilingwateroverthe
herbaldrug(Anvisa,2010).
Herbalproductsareeasilyavailableandwidelycommercialized
(Zhaoetal.,2013;Owensetal.,2014),thereforebeingawayof
com-plementingtraditionalmedicineallovertheworld(Owensetal.,
2014).However,thosearenotcompletelyfreeofpossibletoxicity
orotheradverseeffects(DeSmet,2004).
Theinfusionof CissampelossympodialisEichler,
Menisperma-ceae,speciesiswidelyusedbyIndiantribesandfolkmedicineto
treatvariousinflammatorydisorders,includingasthma
(Bezerra-Santosetal.,2004;Costaetal.,2008;Rochaetal.,2010;Marinho etal.,2012; Cavalcantietal.,2013; Vieiraet al.,2013).Asthma
∗ Correspondingauthor.
E-mail:liane.franco@ccs.ufpb.br(L.F.B.Mangueira).
isamedical conditionwhichpresentsasmain
physiopathologi-calcharacteristicbronchialinflammation,accompaniedbylower
airwayhyperresponsivenessandvariableairflowlimitation.This
inflammationisassociatedwithsevereleukocyterecruitmentand
theiractivationatthesiteoflesion(Bezerra-Santosetal.,2012;
Cavalcantietal.,2013;Ribeiro-Filhoetal.,2013).
The species is endemic in the Northeast and Southeast of
Brazil, frequently occurring in open areas, as shrubs in sandy
soil(Barbosa-Filhoetal.,1997).Theplantispopularlyknownas
“milona”,“jarrinha”,“orelha-de-onc¸a”and“abuteira”(Agraetal.,
2007a,b).
Severalstudies wereconducted withthis plant,which have
proveditstherapeuticpotential(Cavalcantietal.,2013).Studies
revealedanti-inflammatoryactivityandthepotentialfor
modulat-ingthemicrobicideactivityofmacrophagesbyincreasingtheIL-10
productionalongwithinhibitionofNOsynthesis.Furthermore,the
findingsprovedtheefficacyofC.sympodialisupontheregulation
ofBcellfunctionandtheimmunoglobulinsecretioninallergic
dis-eases,aswellasautoimmunediseasesynthesis(Cavalcantietal.,
2013;Vieiraetal.,2013;Piuvezametal.,2012).
http://dx.doi.org/10.1016/j.bjp.2015.06.009
492 L.F.B.Mangueiraetal./RevistaBrasileiradeFarmacognosia25(2015)491–498
Vieiraetal.(2013)demonstratedthattheinhalationofC. sym-podialisinanimalswithallergicinflammationoftheairwaysisas
effectiveastheoraltreatmentwithdexamethasoneforcontrolling
theinflammatoryresponseinthelungsandtheproductionofIgE.
Ultimately,theresultssuggestthattheleavesofC.sympodialismay
bematerialforaherbalmedicine.
Inpre-clinicaltoxicologicalassayswiththeAFL(alcoholic
frac-tionofleaves)ofC.sympodialisdonein rats(maleand female),
itwasinvestigatedthesub-acute(fourweeks)andchronicle
(thir-teenweeks)toxicityofthepopularadministration(9mg/kg/orally).
Thesestudiessuggestedlackoftoxicityin theseanimals.Doses
administrated5–225timeshigherthantheonesingestedbymen
evidenced,inmice,inflammatoryprocessesandanincreaseof
hep-aticenzymes,besidesthehyperplasiaofKupffercells,reversible
thirtydaysaftertheadministrationoftheextractwassuspended
(Diniz,2000).
Datapublishedinpre-clinicalstudieswithC.sympodialisleaves
enableclinicalassayswhichmayinitiallyestablishthesafetyand
subsequentlytheeffectivenessofC.sympodialisinhumans.Thus,
thisstudyintendedtoascertainthesafetyofthismedicinein
poten-tial.Basedonclinicalphase1parametersinconjunctionwiththe
onesinthepre-clinicalstudieswhichhavealreadybeenpublished,
thereis cravingfor registeringwithAnvisa(CNS,1997; Anvisa,
2010).
Materialandmethods
Plantmaterial
TheleavesofCissampelossympodialisEichler,Menispermaceae,
werecollectedduringthemonthsofMarchandSeptemberof2012
inthegardenofmedicinalplantsattheLaboratoryof
Pharmaceu-ticalTechnologyProf.DelbyFernandesdeMedeiros,CampusIat
FederalUniversityofParaíba,wheretheplanthasbeencultivated.
Theidentificationandmorphologicaldescriptionoftheplantwere
madebyDra.MariadeFátimaAgra.AsampleislocatedintheLauro
PiresXavierherbarium,atUFPB,bythevoucherspecimennumber
Agra1456(JPB).
TheacquisitionofC.sympodialissachets
TheleavesofC.sympodialisweredehydratedinagreenhouse
withair flowing at 38◦C for 72hand ground in a Harley type
grinder,havingtheaverageyieldscalculated.Afterthepounding,
thedriedleavesweresubmittedtoaphytochemical/quality
con-troltriage,andthensenttotheAplafLtda,SãoPaulo-SP,beingthis
companyresponsibleforproducingandratifyingthequality
con-troloftheC.sympodialissachets.Eachunit,producedwithfilter
paperforspecificuse,contained1gofthepowder.
Phytochemical
AphytochemicaltrialofC.sympodialisleavesinfusionwas
con-ductedaccordingtoMatos(1997).Duringthetrial,thepresenceof
alkaloids,steroids,tannins,flavonoidsandsaponinweredetected.
TheclassesofchemicalsubstancespresentontheleavesofC.
sympodialiswerecharacterizedbeforethesachetsproduction,thus
beingconsideredanindispensablestagefortheirstandardization.
Searchofwarifteineandmethylwarifteinealkaloidsinteas sachetsofC.sympodialis
Theteaswerepreparedunderthesameconditionsofclinical
studies(onesachetcontaining1gofthepowderC.sympodialiswas
subjectedtoinfusionfor15min).
HPLCequipmentandconditions
Allsolvents used were HPLC level. DeionizedMilli Q water
(Millipore,Bedford,MA) wasusedtopreparethemobilephase
and diluents solutions. All chromatographic runs were carried
outusingaSykamHPLCSystem,consistingofaS7131pump.A
S3240photodiode-arraydetector(DAD)wasusedfordetection.
Fullspectrawererecordedintherange200–400nm.Equipment
control,dataacquisitionandintegrationwereperformedwith
Clar-itysoftware. Chromatographicseparations wereachievedusing
methodologiesbasedonAragão(2002)andMarinho(2011).The
mobilephaseconsistedofamixtureofmethanol/CH3CO2H0.1%
(35:65,v/v). Flow-ratewasset to0.3ml/min and the injection
volumewas20l.Allexperimentswerecarriedoutatroom
tem-perature.TheDADdetectedthepresenceornotofalkaloidspeaks
insachetsofC.sympodialis.ItwasobtainedspectrumUVof
alka-loidtemplatesofC.sympodialis(warifteineandmethylwarifteine)
withinthesameconditionsproposedbythemethodandsavedon
adatabasesupportedbytheClaritysoftware.Thesubstanceswere
analyzedthroughHPLC/UV-DADandbycomparingthetime
reten-tionintheextractpeakswiththeonescollectedwithauthentic
referencestandards.
Researchfield
ThisresearchtookplaceinLauroWanderleyUniversityHospital
atFederalUniversityofParaíba,wherefurthertrialsweredonein
theclinicalanalysislaboratory,inthecardiologyroomaswellas
intheambulatoryatCRAS(ReferenceServerCareCenter).Protocol
clinicwasconducted/definedaccordingtotheBrazilianresolutions
n◦ 251/97and466/12fromtheCNS,theinternationalstandards
fromtheWorldHealthOrganization2011andgoodclinicalpractice
(GCP)(CNS,1997;CNS,2012).
Volunteers
Theclinicalstudywasopenand notrandomized,performed
withindividualsparticipatingvoluntarily.Thesampleconsistedof
eighteenvolunteers,sixmenandelevenwomen(onequitclaim)
between23and60years old,werechosenaftercomplete
clini-calandlaboratorialtrialswhichaimedtoascertainproperhealth
conditionsinordertoparticipateinthisresearch.
Experimentalprotocol
Thevolunteershada150mldoseoftheherbalproductorally,
onceaday,usingthesachetswithleavesofC.sympodialisforfour
weeks.Thus,thisstudywasconductedfromMay2012toJune2013.
Afterreceivingthesachets,theparticipantswereweeklymonitored
(0-1-2-3-4weeks),startingfromday0.
Beforethefirstingestionoftheproductandadayafterthelast
one,theparticipants(menandwomen)weresubjectedtoa
clini-calandlaboratorialevaluationfortheiroverallconditions.Theydid
thefollowingtests:glucose,creatininephosphokinase(CPK),
tria-cylglyceride,totalcholesterolandfractions,lactatedehydrogenase
(LDH),amylase,sodium,potassium,aspartatetransaminase,
ala-ninetransaminase,totalbilirubinandfractions,gammaGT,alkaline
phosphatase,totalproteinandfractions,creatinine,uricacid,urea,
completebloodcount,plateletcountandurinalysisI.Atwelve-lead
electrocardiogramwasalsodone.
Throughout the course of the study, the volunteers were
instructedtoreporttotheresearchersanysignsorsymptomsthat
mightpresentadversereactionandwerealsogivenaquestionnaire
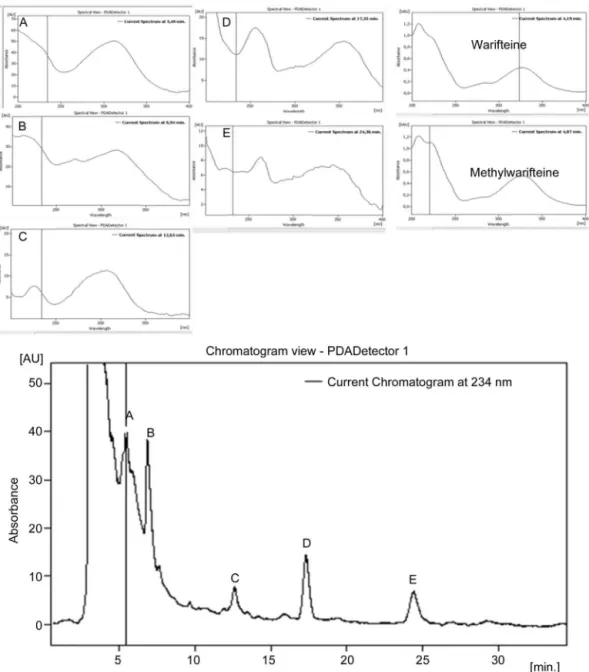
Fig.1. ThechromatogramandUVspectraoftheseparationofteaofCissampelossympodialis.Fiveunknownpeaksareeluting:A,B,C,DandE.
Exclusioncriteria
Individualswho hadanyclinicalorlaboratorialalterations–
hepatic, renal or cardiac dysfunction; pregnancy; use of
alco-holand/oranymedicines–duringtheinitialclinicaltestswere
excludedfromthisstudy.
Ethicalaspects
Theresearchproject,withtheprotocolandconsentforms,was
submittedandapprovedbytheCommitteeofEthicsinResearch
withhumansatLauroWanderleyUniversityHospital–UFPBon
25/05/2010–protocolno.284/10.
Allvolunteerswereinformedaboutthenatureandobjectives
ofthestudyandthosewhoagreedtoparticipategavetheirformal
writtenconsentaftersigningtheStatementofInformedConsent.
Statisticalanalysis
Theevaluationofthevolunteers’hematologicalandbiochemical
parameters,whichaimedtodiagnosewhethertheparticipantsmet
thestandardsforanindividualconsideredhealthy,wasperformed
bycomparingtheresultsobtainedthroughoutthetreatmentand
theonesacquiredduringtheinitialtrialforeachvolunteer(basal
time). The figures wereexpressed by mean±standard error of
mean(SEM)oftheseventeenparticipants,separatingthemby
gen-der,accordingtothetype of testand evaluation period.It was
usedtheStudent’s“t”testforpairedsamples*p<0.05andOne-way
ANOVA/Tukey.*p<0,05.Alldatawereanalyzedusingthestatistical
programGraphPadPrism®version6.02.
Resultsanddiscussion
Thesearchforwarifteineandmethylwarifiteineinteasprepared
withC.sympodialissachetswereperformedthroughanalysisby
HighPerformanceLiquidChromatographycoupledtoUV
detec-torwithphotodiodearray(HPLC/DAD).TheHPLCchromatogram,
Fig.1,inC.sympodialisteashowedfiveunknownpeaksdefinedas:
A,B,C,D,Eandtheabsenceofwarifteineandmethylwarifteinein
measurableconcentrations.Thisfactcanbeexplainedbecause
pre-viousstudiesusedethanolicextraction.Thisstudyusedhotwater
494 L.F.B.Mangueiraetal./RevistaBrasileiradeFarmacognosia25(2015)491–498
Table1
Patients’biochemicalparameters,eithersex,fromtheClinicalTrialwithinfusionoftheleavesofCissampelossympodialis.Valuesareexpressedinmean±SEM.
Groups Glucose(mg/dl) Totalbilirubin (mg/dl)
Directbilirubin (mg/dl)
Indirect bilirubin(mg/dl)
Uricacid(mg/dl) Creatinine(mg/dl) Urea(mg/dl)
Men
Reference 70–99 0.2–1.2 0.0–0.5 0.0–0.5 3.5–7.0 0.9–1.3 19.0–44.0 1◦Appointment 89.67±1.76 0.74±0.23 0.21±0.06 0.54±0.17 6.05±0.43 1.03±0.07 29.50±3.15
30Days 90.33±2.17 0.96±0.34 0.26±0.07 0.70±0.27 6.03±0.49 1.03±0.07 28.33±2.08
Women
Reference 70–99 0.2–1.2 0.0–0.5 0.0–0.5 2.5–6.2 0.6–1.1 14.9–40 1◦Appointment 87.55±1.97 0.63±0.15 0.16±0.03 0.46±0.12 4.23±0.34 0.73±0.03 27.20±2.39
30Days 90.55±4.10 0.64±0.16 0.18±0.03 0.46±0.13 4.25±0.34 0.76±0.04 26.33±2.84 Student’s“t”test*p<0.05probability.
Table2
Patients’biochemicalparameters,eithersex,fromtheClinicalTrialwithinfusionoftheleavesofCissampelossympodialis.
Groups AST(U/l) ALT(U/l) GGT(U/l) Totalphosphiniccreatine(U/l) LDH(U/l) Amylase(U/l)
Men
Reference 5–34 6–55 12–24 <190 125–243 25–125 1◦Appointment 25.17±3.11 42.50±8.96 47.33±10.67 122.00±18.18 65.80±4.42 79.50±13.71
30Days 25.17±3.03 47.33±12.65 47.33±14.78 120.00±24.45 186.00±18.51 78.33±13.07
Women
Reference 5–34 6–55 9–36 <167 125–243 25–160 1◦Appointment 21.40±4.10 25.70±6.55 23.90±5.65 97.50±15.99 153.80±4.35 67.30±3.17
30Days 19.40±2.11 22.40±3.40 22.85±3.51 103.5±11.05 156.00±4.79 73.43±5.07 AST,aspartateaminotransferase;ALT,alanineaminotransferase;GGT,Gamma-glutamyltranspeptidase;LDH,lactatedehydrogenase.
Valuesareexpressedinmean±SEM.Student’s“t”test*p<0.05probability.
Table3
Patients’hematologicalparameters,eithersex,fromtheClinicalTrialwithinfusionoftheleavesofCissampelossympodialis.
Groups RBC(106/mm3) Hemoglobin(g/dl) Hematocrit(%) MCV(fl3) MCH(pg) MCHC(%) RDW(%)
Men
Reference 4.5–6.0 12.8–17.8 40–52 82–98 27–33 32–36 11.5–14.5 1◦Appointment 5.33±0.10 15.72±0.46 45.90±0.99 86.13±1.44 29.51±0.91 34.24±0.52 10.63±0.33
30Days 5.32±0.13 15.76±0.29 46.06±1.01 86.65±1.01 29.68±0.55 34.26±0.33 10.92±0.15
Women
Reference 3.9–5.3 12–15.6 36–48 82–98 27–33 32–36 11.5–14.5 1◦Appointment 4.42±0.09 13.33±0.23 39.02±0.68 88.31±1.19 30.20±0.52 34.17±0.22 11.22±0.34
30Days 4.38±0.10 13.22±0.23 38.51±0.61 88.07±1.04 30.25±0.61 34.34±0.38 11.38±0.38 RBC,redbloodcells;MCV,meancorpuscularvolume;MCH,meancorpuscularhemoglobin;MCHC,meancorpuscularhemoglobinconcentration;RDW,redcelldistribution width.
Valuesareexpressedinmean±SEM.Student’s“t”test*p<0.05probability.
This study investigated the clinical toxicity in seventeen humans,insearchofthesafetyofthismedicineinpotential.The clinicalphaseIconcernsthefirstmomentwhenthemedicineis testedinagroupofhealthyvolunteers.Thisphaseseekstoestablish thesafety,pharmacokineticprofileandtolerabilityinapreliminary formatofthesubstanceinhumans(CNS,1997;Mesiaetal.,2011).
TestswerecarriedoutforurinalysisI,hematologicaland
bio-chemicalparametersofpatients,whichshowedthattherewasjust
asignificantchangeinstatisticsinfourhematologicalparameters,
inwhichitwasobservedadeclineinleucocytesandneutrophils
inmen,anincreaseineosinophilsinwomenandinlymphocytes
inmen.Althoughthesechangeswerestatisticallysignificant,they
donotrepresentapathologicalcondition,andperhapsarejustan
alterationofnormalhumanphysiology(Tables1–4).
Theleucocytesandlymphocytesarecellsresponsiblefor
pro-tectingtheorganismagainstinfections.Lymphocytosisiscaused
byneoplasia,viralinfectionssuchasrubella,mononucleosis,and
mumps;bacterialinfections,protozoans,amongothers(Hoffbrand
andMoss,2013;Mussoetal.,2014).Theneutrophilsaremainly
responsibleforthephagocytesisofcellsandextraneousmaterial.
Neutropeniaismainlycausedbydysplasia,infections,
inflamma-tion, intravascular destruction (immune), drugs and chemicals,
amongothers(Mussoetal.,2014;Spaanetal.,2013).Ontheother
hand,the eosinophiliais related toallergic responses,parasitic
Table4
Patients’hematologicalparameters,eithersex,fromtheClinicalTrialwithinfusionoftheleavesofCissampelossympodialis.
Groups Leukocytes(103/mm3) Neutrophils(%) Eosinophils(%) Lymphocytes(%) Monocytes(%) Platelet(1/mm3)
Men
Reference 4.0–11.0 45–70 1–6 20–45 2.0–10.0 150,000–450,000 Basal 9265±718.40 58.83±3.08 2.83±0.60 30.83±3.12 7.5±0.68 207717±31716 30Days 8293±636.10 53.67±2.33 3.00±0.37 35.67±2.14 7.5±0.56 237683±23855
Women
0 20 40 60 80 100
Car
diac
fr
e
q
u
e
n
cy
(bea
ts
/m
in
)
basal first week
second weekthird weekfourth week basal
first week
second weekthird weekfourth week basal
first week
second weekthird weekfourth week
basal first week
second weekthird weekfourth week
basal first
wee k
seco nd w
eek
third wee k
fourth wee k basal
first week
second weekthird weekfourth week
0 20 40
30
20
10
0
30
20
10
0 40
30
20
10
0
Respiratory
frequency (breaths/min)
Temperature (ºC)
Body Mass Index (weight/height
2)
60 80 150
100
50
0 Dia
s
tol
ic
bl
oo
d
pr
e
ssu
re
(m
m
H
g
)
Systolic
bl
oo
d
pr
e
ssu
re
(m
m
H
g
)
Fig.2. Weeklyevaluationofwomenaccordingtothefollowingparameters:temperature,respiratoryandcardiacfrequency,systolicanddiastolicbloodpressureandbody massindex.Valuesareexpressedinmean±SEM.One-wayANOVA/Tukey.*p<0.05.
diseases, acuteinfection, certainskin diseases, drugsensitivity, amongothers(Chenetal.,2013;Dasguptaetal.,2013;Mussoetal.,
2014).Thesepathologieswerenotdetectedinthevolunteerswho
participatedinthestudy.
Withinthiscontext,thelowtoxicityofC.sympodialiswas
ver-ified,sinceitwasnotrevealedanysignificantstatisticalchanges
inthevolunteers’biochemicaltests,despitethefactthatstudies
demonstratehepaticalterationsinhumansthroughout
continu-oususeofmedicinalplants(Pauloetal.,2009;Bunchorntavakul
andReddy,2013).Furthermore,whencomparingtheresultsfrom
thepre-clinicaltrialstotheclinicalones,werealizedthattheremay
besomestatisticalvariation,yetonlyinoverdose(Diniz,2000).
Alongtheclinicaltests,anamnesis,temperaturemeasurement,
blood pressureinvestigation,respiratory and cardiacfrequency,
bodymassindexandapplicationofquestionnairesrelatedto
pos-sibleside andadverse effects wereconductedby thephysician
responsiblefortheresearch,whoevaluatedand diagnosedthat
theindividuals involvedin thestudywerein normalstandards
(Figs.2and3).Theelectrocardiogramsshowedelevennormalones
496 L.F.B.Mangueiraetal./RevistaBrasileiradeFarmacognosia25(2015)491–498
basal first week
second weekthi rd week
fourth week basal
first week second weekthi
rd week fourth week
basal first week
second weekthi rd week
fourth week
basal first week
second weekthi rd week
fourth week
basal first week
second weekthi rd week
fourth week basal
first week second weekthi
rd week fourth week
0 20 40 60 80 100
Temperature (ºC)
Respiratory frequency
(breaths/min)
Cardiac frequency (beats/min)
40
30
20
10
0
30
20
10
0
150
100
50
0
100
80
60
40
20
0
40
30
20
10
0
Diastolic blood pressure
(mmHg)
Body Mass Index (weight/height
2)
Systolic blood pressure
(mmHg)
Fig.3.Weeklyevaluationofmenaccordingtothefollowingparameters:temperature,respiratoryandcardiacfrequency,systolicanddiastolicbloodpressureandbody
massindex.Valuesareexpressedinmean±SEM.One-wayANOVA/Tukey.*p<0.05.
oftherightbundlebranchbeforeandafter;oneshortPRbefore
andafter; onebradycardicbeforeand after;one normalbefore
andonealteredventricularrepolarizationafter;onebradycardic
beforeand1alteredventricularrepolarizationafter.Nevertheless,
allelectrocardiographictestswereasexpectedbasedonnormal
limits.
Theelectrocardiogram(ECG)isasimpleandlowcosttestwhich
providesdata about the myocardium status, perhaps useful in
cardiovascularepidemiology,thereforesupportingthediagnosis
ofthemyocardialinfarction,ischemia andcardiachypertrophy,
andyetdetectingtheriskoffuturecardiacevents(Cardosoetal.,
2002;Ribeiroetal.,2012).
However,thenormal rangeof theelectrocardiogramis
con-troversial, hence it demands a thorough analysis of variations
consideredexpectedintheECGofhealthypeople,and,onlyafter,
Table5
Sideeffectsandadversereactionsdescribedthroughouttheclinicalstudy.
Effects Numbers Frequency(%) Fluidretention 1/17 5.88 Decreasedmuscleaches 2/17 11,76 Hydrationofheels 1/17 5.88 Decreasedappetite 5/17 29.41 Reductionincandida 1/17 5.88 Decreasetingling 1/17 5.88 Increasedintestinalmotility 2/17 11.76
Dizziness 1/17 5.88
Somnolence 1/17 5.88
Insomnia 1/17 5.88
areundoubtedlyabnormal(MoffaandSanches,2001;Hampton, 2011).
Sinusrhythmis theonlyonesustainedas normal.In youth,
thespaceRR is reduced, in otherwords,thecardiac frequency
isincreasedduringinspirationanditisnamedrespiratorysinus
arrhythmia.Whenthesinusarrhythmiaisintense,itmaysimulate
anatrialone(MoffaandSanches,2001;Hampton,2011).
Thereisnosingledefinitionforanormalcardiacfrequency,and
thetermsbradycardiaandtachycardiaoughttobeusedcautiously.
Thereisnopointatwhichahighcardiacfrequencyinsinusrhythm
shouldbecalledsinustachycardia and noupperlimit forsinus
bradycardia.However, unexpectedhighor low rates shouldbe
investigated.Thepossiblecausesforasinusrhythmoflowcardiac
frequencyare:goodphysicalcondition,hypothyroidism,
hypother-mia, acute myocardial infarction, vasovagal attacksand use of
beta-blockers.Themostcommoncausesdescribedassinusrhythm
of highcardiac frequency is:pain, fear, obesity, acute
myocar-dialinfarction,pulmonaryembolism,anemia,thyrotoxicosis,useof
beta-adrenergicdrugs(MoffaandSanches,2001;Hampton,2011).
Thesepathologieswerenotdetectedinthevolunteerswho
partic-ipatedinthestudy.
StudiesshowedthatsmallchangesintheECGmayberegarded
as“predictive”forclinicalsignsofacoronaryheartdisease,which
isrelatedtocardiovascularmortality(MoffaandSanches,2001;
Hampton,2011).Someauthorssuggestthatanabnormalityinthe
ST-Tsegmentisanindependentindicatorofmorbidityand
mor-tality fromcoronary atherosclerosis (Moffa and Sanches, 2001;
Baranowskietal.,2012).
Forthisreason,theECGhasbeenwidelyusedtoidentify
individ-ualsatriskforisquemicheartdiseases,stillduringasymptomatic
stage. This population, once subjected topreventive and quite
aggressivestrategies,maybebenefited.
Throughouttheclinicalresearch,somesideandadverseeffects
wereobserved,suchas:decreasedmuscleaches;skinhydration;
increasedintestinalmotility;decreasedtinglingsensation.
More-over,dizziness,insomnia,fluidretention,somnolence,reduction
incandidaanddecreasedappetitewerealsoreportedassideand
adverseeffects(Table5).
TheseadversereactionsmaybeclassifiedTypeA,referredtoas
toxicorsideeffects,whichcanbeexplainedbytheaction
mecha-nismofdrugs,beingacommonandexpectedreactionandoflow
mortalityrate(EdwardsandAronson,2000;Sobrafo,2011).
Thedatacollectedfromthisstudydemonstratedthatthe
admin-istration of C. sympodialis leaves infusion in humans, ingested
duringthirtydays,waswelltolerated,indicatingneitherclinical
norlaboratorialalterations,noranysignificantadversereactions.
Theseresultscomplementthoseobtainedinthepre-clinical
toxi-cologicalstudies,suggestinglowtoxicity,inthedosageandroute
ofadministrationtested.Withthisdata,thereiscravingfor
regis-teringthismedicineinpotentialwithAnvisaandproceedwiththe
phaseIIstudies.
Authors’contributions
LFBM(PhDstudent)mainauthor,involvedinthestudydesign,
conducted the interviews,field and laboratory work,literature
reviewandgeneraldatacollection,systematizationandanalysis,
wrotethefirstdraftthis paper.LSNRandAIPAT, contributedto
designingandfollowingprogressoftheresearchandfieldwork.JAR
andKMO,contributedtoinachievingthelaboratorytestanddata
analyses.CFSA contributedto chromatographic analysis.VMVN
contributedtocardiacmonitoringandreports.ABLandCMBLL,
con-tributedinwritingthefinalversionofthispaper.MFFMDdesigned
thestudy,supervisedthelaboratoryworkandcontributedto
crit-icalreading themanuscript.Alltheauthorshaveread thefinal
manuscriptandapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
References
Agra,M.F.,Freitas,P.F.,Barbosa-Filho,J.M.,2007a.Synopsisoftheplantsknown
asmedicinalandpoisonousinNortheastofBrazil.Rev.Bras.Farmacogn.17, 114–140.
Agra,M.F., Nurit-Silva, K., Baracho, G.S., Basilio,I.J.L.D., 2007b. Estudo
fama-cobotânicodefolhasdeNicotianaglauca(Solanaceae).Lat.Am.J.Pharm.26, 499–506.
Anvisa, 2010. Ministério da Saúde, Agência Nacional de Vigilância Sanitária.
Resoluc¸ãoRDCn◦10,de9demarc¸ode2010.Dispõesobreanotificac¸ãodedrogas vegetaisjuntoàAgênciaNacionaldeVigilânciaSanitáriaedáoutrasprovidências. DiárioOficialdaUnião,Brasília,DF.
Aragão,C.F.S.,2002.Desenvolvimentodemetodologiasanalíticasparapadronizac¸ão
de extratos de Cissampelos sympodialis Eichl(Milona). Tese de doutorado, ProgramadePós-graduac¸ãoemProdutosNaturaiseSintéticosBioativos. Uni-versidadeFederaldaParaíba,JoãoPessoa,210p.
Barbosa-Filho,J.M.,Agra,M.F.,Thomas,G.,1997.Botanical,chemicaland
pharma-cologicalinvestigationonC.sympodialisfromParaíba(Brazil).Cienc.Cult.49, 386–394.
Baranowski,R.,Małek,L.,Prokopowicz,D.,Spiewak,M.,Mi´sko,J.,2012.
Electro-cardiographicdiagnosisoftheleftventricularhypertrophyinpatientswith leftbundlebranchblock:isitnecessarytoverifyoldcriteria?Cardiol.J.19, 591–596.
Bezerra-Santos, C.R., Balestieri, F.M., Rossi-Bergmann, B., Pec¸anha, L.M.T.,
Piuvezam, M.R., 2004. Cissampelos sympodialis Eichl.
(Menisperma-ceae): oral treatment decreases Ige levels and induces a Th1-skewed cytokine productionin ovalbumin-sensitized mice.J. Ethnopharmacol. 95, 191–197.
Bezerra-Santos,C.R.,Vieira-de-Abreu,A.,Vieira,G.C.,Ribeiro-Filho,J.,Barbosa-Filho,
J.M.,Pires,A.L.,Martins,M.A.,Souza,H.S.,Bandeira-Melo,C.,Bozza,P.T.,
Piu-vezam,M.R.,2012.EffectivenessofCissampelossympodialisanditsisolated
alkaloidwarifteineinairwayhyperreactivityandlungremodelinginamouse modelofasthma.Int.Immunopharmacol.13,148–155.
Bunchorntavakul,C.,Reddy,K.R.,2013.Reviewarticle:herbalanddietary
supple-menthepatotoxicity.Aliment.Pharmacol.Ther.37,3–17.
Cardoso,E.,Martins,I.S.,Fornari,L.,Monachini,M.C.,Mansur,A.P.,Caramelli,B.,
2002.Alterac¸õeseletrocardiográficasesuarelac¸ãocomosfatoresderiscopara
doenc¸aisquêmicadocorac¸ãoempopulac¸ãodaáreametropolitanadeSãoPaulo. Rev.Assoc.Med.Bras.48,231–236.
Cavalcanti,A.C.,Melo,I.C.A.R.,Medeiros,A.F.D.,Neves,M.V.M.,Pereira,A.N.,Oliveira,
E.J.,2013.StudieswithCissampelossympodialis:thesearchtowardsthescientific
validationofatraditionalBrazilianmedicineusedforthetreatmentofasthma. Rev.Bras.Farmacogn.23,527–541.
CNS,1997.MinistériodaSaúde,ConselhoNacionaldeSaúde.Resoluc¸ãon◦251,de
7deagostode1997.Contemplaanormacomplementarparaaáreatemática especialdenovosfármacos,vacinasetestesdiagnósticos.DiárioOficialdaUnião, Brasília,DF.
CNS,2012.MinistériodaSaúde,ConselhoNacionaldeSaúde.Resoluc¸ãon◦466,de
12dedezembrode2012.Aprovaasnormasregulamentadorasdepesquisas envolvendosereshumanos.DiárioOficialdaUnião,Brasília,DF.
Costa,H.F.,Bezerra-Santos,C.R.,Barbosa-Filho,J.M.,Martins,M.A.,Piuvezam,M.R.,
2008.Warifteine,abisbenzylisoquinolinealkaloid,decreasesimmediateallergic
andthermalhyperalgesicreactionsinsensitizedanimals.Int. Immunopharma-col.8,519–525.
Chen,L.,Zhong,N.,Lai,K.,2013.Re-challengewithovalbuminfailedtoinduce
bronchialasthmainmicewitheosinophilicbronchitis.PLOSONE8,e75195.
DeSmet,P.A.,2004.Healthrisksofherbalremedies:anupdate.Clin.Pharmacol.
Ther.76,1–17.
Diniz,M.F.F.M.,2000.Ensaiostoxicológicospré-clínicoscomasfolhasde
498 L.F.B.Mangueiraetal./RevistaBrasileiradeFarmacognosia25(2015)491–498
Pós-graduac¸ãoemProdutosNaturaiseSintéticosBioativos.Universidade Fed-eraldaParaíba,JoãoPessoa.
Dasgupta,A.,Neighbour,H.,Nair,P.,2013.Targetedtherapyofbronchitisin
obstruc-tiveairwaydiseases.Pharmacol.Ther.140,213–222.
Edwards,I.R.,Aronson,J.K.,2000.Adversedrugreactions:definitions,diagnosis,and
management.Lancet356,1255–1259.
Hampton,J.,2011.ECGnaprática.Elsevier,Brasil.
Hoffbrand,A.V.,Moss,J.E.,2013.Fundamentosemhematologia.Artmed,Porto
Ale-gre.
Marinho,A.F.,Barbosa-Filho,J.M.,Oliveira,E.J.,2012.Avalidatedmethodforthe
simultaneousquantitationofbioactivealkaloidmarkersintheleaf ethano-licextractofCissampelos sympodialisEichl: aphenological variationstudy. Phytochem.Anal.23,426–432.
Marinho,A.F.,2011.Caracterizac¸ãodosmarcadores,desenvolvimentoeavaliac¸ãode
métodoanalíticoaplicadoaoestudodesazonalidadeeidentificac¸ãodenovos alcaloidesdeCissampelossympodialis.Tesededoutorado,Programade Pós-graduac¸ãoemProdutosNaturaiseSintéticosBioativos.UniversidadeFederal daParaíba,JoãoPessoa,192p.
Matos,F.J.A.,1997.Introduc¸ãoaFitoquímicaExperimental.Edic¸õesUFC,Fortaleza.
Mesia,K.,Cimanga,K.,Tona,L.,Manpunza,M.M.,Ntamabyaliro,N.,Muanda,T.,
Muyembe,T.,Totté,J.,Met,T.,Pieters,L.,Vlientinck,A.,2011.Assessmentof
theshort-termsafetyandtolerabilityofaquantified80%ethanolextractfrom thestembarkofNaucleapobeguinii(PR259CT1)inhealthyvolunteers:aclinical phaseIstudy.PlantaMed.77,111–116.
Moffa,P.J.,Sanches,P.C.R.,2001.Eletrocardiograma:normalepatológico.Roca,
(SérioInCor)SãoPaulo.
Musso,A.,Catellani,S.,Canevali,P.,Tavella,S.,Vene,R.,Boero,S.,Pierri,I.,Gobbi,
M.,Kunkl,A.,Ravetti,J.L.,Zocchi,M.R.,Poggi,A.,2014.Aminobisphosphonates
preventtheinhibitoryeffectsexertedbylymphnodestromalcellsonanti-tumor V␦2Tlymphocytesinnon-Hodgkinlymphomas.Haematologica99,131–139.
Owens,C.,Baergen,R.,Puckett,D.,2014.Onlinesourcesofherbalproduct
informa-tion.Am.J.Med.127,109–115.
Paulo,P.T.C.,Diniz,M.F.F.M.,Medeiros,I.A.,Morais,L.C.S.L.,Andrade,F.B.,Santos,
H.B.,2009.Ensaiosclínicostoxicológicos,faseI,deumfitoterápicocomposto
(SchinusterebinthifoliusRaddi,PlectranthusamboinicusLoureEucalyptusglobulus Labill).Rev.Bras.Farmacogn.19,68–76.
Piuvezam,M.R.,Bezerra-Santos,C.R.,Bozza,P.T.,Melo, C.B.,Vieira,G.C.,Costa,
H.F., 2012. Cissampelos sympodialis (Menispermaceae): a novel
phy-totherapic weaponagainst allergicdiseases? In:Pereira, C. (Ed.),Allergic
Diseases – Highlights in the Clinic, Mechanisms and Treatment. InTech,
ISBN 978-953-51-0227-4, http://dx.doi.org/10.5772/25739, Available from:
http://www.intechopen.com/books/allergic-diseases-highlights-in-the-clinic- mechanisms-and-treatment/cissampelos-sympolialis-menispermaceae-a-
novel-phytotherapic-weapon-against-allergic-diseases-Ribeiro-Filho,J.,Calheiros,A.S.,Vieira-de-Abreu,A.,Carvalho,K.I.,Silva-Mendes,
D.,Melo,C.B.,Martins,M.A.,Silva-Dias,C.,Piuvezam,M.R.,Bozza,P.T.,2013.
Toxicol.Appl.Pharmacol.273,19–26.
Ribeiro,S.M.,Morceli,J.,Gonc¸alves,R.S.,Franco,R.J.,Habermann,F.,Meira,D.A.,
Matsubara,B.B.,2012.Accuracyofchestradiographypluselectrocardiogramin
diagnosisofhypertrophyinhypertension.Arq.Bras.Cardiol.99,825–833.
Rocha,J.D.,Decoté-Ricardo,D.,Redner,P.,Lopes,U.G.,Barbosa-Filho,J.M.,
Piu-vezam,M.R.,Arruda,L.B.,Pec¸anha,L.M.T.,2010.Inhibitoryeffectofthealkaloid
warifteinepurifiedfromCissampelossympodialisonBlymphocytefunction invitroandinvivo.PlantaMed.76,325–330.
Spaan,A.N.,Surewaard,B.G.,Nijland,R.,Strijp,J.A.,2013.Neutrophilsversus
Staphy-lococcusaureus:abiologicaltugofwar.Annu.Rev.Microbiol67,629–650.
Sobrafo, 2011. Guia para Notificac¸ãode Reac¸ões Adversas em Oncologia. In:
SociedadeBrasileiradeFarmacêuticosemOncologia.ConectfarmaPublicac¸ões Científicas,SãoPaulo.
Vieira,G.C.,DeLima,J.F.,DeFiguereido,R.C.,Mascarenhas,S.R.,Bezerra-Santos,C.R.,
Piuvezam,M.R.,2013.InhaledCissampelossympodialisdown-regulatesairway
allergicreactionbyreducinglungCD3+Tcells.Phytother.Res.27,916–925.
Zhao,J.,Deng,J.W.,Chen,Y.W.,Li,S.P.,2013.Advancedphytochemicalanalysisof