First-Priniples Study of the Adsorption
of PH
3
on Ge(001) and Si(001) Surfaes
R.Miotto, A. C. Ferraz,
InstitutodeFsiadaUniversidadedeS~aoPaulo,
CaixaPostal66318,CEP05315-970,S~aoPaulo,SP,Brazil
and G.P.Srivastava
Shoolof Physis,Universityof Exeter,StokerRoad,ExeterEX44QL,UnitedKingdom
Reeivedon23April,2001
Usingarst-priniplespseudopotentialmethodwehaveomparedtheadsorptionanddissoiation
of the ommon n-type dopant moleule PH
3
on the Si(001)-(21) and Ge(001){(21) surfaes.
We nd that the dissoiated state is energetially more favourable than the moleular state by
1.70(0.81) eV, whereas the latter is 0.58(0.25) eV morestable thanthe system omposed of the
freesilion(germanium)surfaeandPH3(g). Thehemisorbedsystemisharaterisedbyelongated
Si{Si(Ge{Ge) dimersthataresymmetriinthedissoiativease andasymmetriinthemoleular
ase and by the fat that the Si(Ge){PH
2
as well as the PH
3
(ads) groupsretain the pyramidal
geometryofthephosphinemoleule. Ourdissoiativeadsorptionmodelisfurthersupportedbyour
alulated vibrationalmodes,whihareingoodagreementwithavailableexperimentalworks.
Preise ontrol of doping is importantfor the
fab-riation of novel semiondutor devies with
dereas-ing dimensions. Due to its hemial simpliity,
phos-phine gas is a very ommon in situ n-type dopant
soure in hemial vapour deposition (CVD) or
gas-souremoleular-beamepitaxyofSiGelms[1,2℄. One
oftheproblemsrelatedwiththeuseofphosphineasa
dopantistheresultantdereaseofthegrowthrate,
de-grading the throughput of the devie proessing. In
order to eluidate this issue, the interation of
phos-phinewiththesilionandgermaniumsurfaeshasbeen
the fous of many experimental works [1-7℄,involving
dierent tehniques. Due to its tehnologial
impor-tane in the prodution of ultra-large-saleintegrated
iruits,the interation proess ofphosphine withthe
silionsurfaeonentratesthemajorityof
experimen-tal studies sofar. Inageneral sense theexperimental
resultssuggestthatphosphineismoleularlyadsorbed
ononesideofaSi(orGe)dimeratroomtemperature.
ForlowPH
3
overages[0:18monolayers(ML)℄,the
moleuledissoiatesintoPH
2
andH,withPH
2
andHat
dierentsidesoftheSi orGedimer. Forhigher
over-ages,onlyasmallamountofPH
3
dissoiatesduetothe
lakofemptydanglingbonds. Theexperimental
obser-vationofmoleularadsorptionontheSi(001)surfaeis
alsoorroboratedby available theoretialworks[5,8℄.
tionof phosphineontheGe(001)surfaeareavailable
sofar. Despitethegreatnumberofexperimentalworks
andsometheoretialeortsfortheinterationof
phos-phinewith thesilionsurfae,littleis knowabout
de-tailsoftheadsorption,dissoiationandloalgeometry
resulting from the interation of phosphine with the
germanium surfae. Thisinformationis essentialfora
ompleteunderstandingofthedopingmehanism.
The surfae was modelled in a super-ell
geome-try, with an atomi slab of eight Si or Ge layersand
avauumregionequivalenttoeigthatomilayers. The
theoretial bulk lattie onstant of 5.42
A for Si and
5.74
AforGewasusedinthesurfaealulations. On
thetopsideoftheslabweplaedthePH
3
moleulein
dierent ongurations,andthebaksurfaewas
pas-sivated byH atoms arrangedin adihydride struture.
ThepseudopotentialsforGe,Si,P,andHwerederived
by using the sheme of Troullier and Martins [9℄ and
the eletron-eletron exhange orrelation was
onsid-ered by using GGA [10℄. The single-partile orbitals
wereexpressed inaplane-wavebasisupto thekineti
energy of 16 Ry and four speial k points were used
fortheBrillouin-zonesummation. Theatomswere
as-sumed to be in their fully relaxed positions when the
foresatingontheionsweresmallerthan0.005eV/
sorbed state, and from the adsorbed state to the
dis-soiated state was obtainedby using aonstraint
dy-namis sheme in whih atomi movement along the
pathwayisaresponsetoforesinaloalregionaround
it. Thevibrationalmodeswerealulatedbyusingthe
frozen-phononapproah. Inouralulationsforthe
dy-namial propertiesonly the adsorbed system(PH
3 or
PH
2
+H)andtheSiorGedimeratomswereallowedto
vibrate.
Itisnowwellstablishedthat,atlowtemperatures,
the (001) surfae of silion and germanium exhibits a
(42)reonstrution. Thishangestoa(21)
reon-strution at roomtemperature,thereforethe majority
ofexperimentsontheadsorptionofphosphineare
per-formed on the Si(001) or Ge(001){(21)
reonstru-tions (seefor exampleRef. [4, 5℄). Forthis reason we
willonlyonsiderthe(21)reonstrutionthroughtout
thispaper.Wehavedeterminedtheequilibrium
geome-triesofthephosphineadsorbedSiandGe(001)surfaes
for the moleular (i.e. PH
3
bonded at oneside of the
Si(Ge) dimerasin Fig.1(a))anddissoiated(i.e. PH
2
and H bonded to dierent omponents of the dimer
as in Fig.1(b)) models. The bridgeonguration(i.e.
PH
3
bonded to both Si(Ge) atoms onthe Si{Si (Ge{
Ge)dimer)earlyproposedbyWangando-workers[3℄
will notbe disussed in this work aswehave already
shownthat it doesnot orrespondto astable
adsorp-tiononguration[8℄.
θ
φ
ω
θ
ω
[110]
[001]
(a) molecular
(b) dissociated
Si (Ge)
P
H
Figure1. Shematisideviewofthemoleularand
dissoi-atedadsorptionmodelsforPH3onX(001){(21),whereX
=SiorGe.
We have found that the dissoiative adsorption
model, shown in Fig. 1(b), is energetially more
favourable than the moleular state (Fig. 1(a)) by
1.70eVforSi and0.81eVforGe. Ourrst-priniples
alulations also show that the moleularly adsorbed
systemis 0.58eV forSi and 0.25eVforGe more
sta-ble than the systemomposed of the freesurfaeand
PH
3
(g), suggestingthatthemoleularly adsorbed
sys-tem orrespondsto ameta-stable onguration. This
moleular adsorption followed by the dissoiation
un-derlow overageexposure(see, for example,Ref. [7℄).
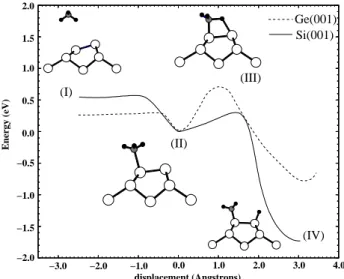
InFig.2wepresenttheenergydiagramobtainedfora
probabledissoiativepathwayonsidering alower
ov-erage adsorption. Along the path from the gas-phase
PH
3
(I) to the moleularly adsorbed state (II), there
is no adsorption barrier. This spontaneous proess is
mainly due to the energetially favourable
hybridiza-tionbetweenthelone-pairstateofPH
3
andtheempty
danglingbondstateofthedownatom ontheSi orGe
dimer. Theenergy barrierfor theadsorbed moleular
phasePH
3
(II) to dissoiateintoPH
2
andH(IV) was
foundtobe0.31eVforSibut0.72eVforGe.Thepeak
of the dissoiation barrier orresponds to atransition
staterepresentedinFig.2(III).
0.0
1.0
2.0
3.0
4.0
displacement (Angstrons)
1.5
2.0
1.0
0.5
−1.0
−1.5
−2.0
−0.5
0.0
Ge(001)
Si(001)
(I)
(III)
(II)
(IV)
Energy (eV)
−3.0
−2.0
−1.0
Figure2. Calulated energybarrier diagramfor the
disso-iativeadsorptionproessofPH
3
onX(001){(21),whereX
=SiorGe: (I)orrespondsto X(001){(21)+PH
3 (gas);
(II) orresponds to X(001){(21) + PH
3
(ads) (moleular
adsorption); (III) orresponds to the transition state and
(IV) orresponds to X(001){(21) + PH
2
(ads) + H(ads)
(dissoiativeadsorption)
Consideringthephenomenologial approah in the
formoftheArrheniusequation[11℄,withthehoiefor
theA-fatorbetween10 13
10 15
s 1
,wehavestimated
that,forompletedissoiation,anativationbarrierof
0.31eV(0.72eV)orresponds toathermalativation
intherange104{120K(241{278K).Thisisonsistent
withtheexperimentaldissoiationofPH
3
onthesilion
surfaeevenat100K[4℄observedatlowoverage.Our
resultsalsoindiates that, forthegermanium surfae,
the phosphine moleule dissoiates at room
tempera-ture. However, as show in Fig. 2, for the Ge based
system desorption is more favourable than the
disso-iation proess. We would like to re-emphasise that
a great number of dierent pathways maybe allowed
duringthedissoiationofthemoleule.
InTable1wepresentouralulatedstrutural
pa-rametersobtainedforthemoleularanddissoiated
ad-sorptionmodelsofPH
3
onSi(001)andGe(001){(21)
as well as some available theoretial data [5℄ for the
silion based system. Our results are in good
agree-ment with the rst-priniples alulations by Kipp et
al [5℄. Upon the adsorption of phosphine, while for
the silion based system the dimers get elongated by
6%,forthegermaniumbasedsystem,weobservethat
the dimer elongation is muh smaller: 2%. In both
ases, forthe moleular adsorption theSi{Si and Ge{
Ge dimer retains its asymmetri onguration, while
forthe dissoiated systemitbeomesalmost
symmet-ri. ForthemoleularmodelwealulatedaSi{P(Ge{
P)bondlengthof2.35
A(2.49
A)inontrasttoamuh
smaller valueof 2.27
A (2.38
A) obtainedfor the
dis-soiated model. The weakening of the Si{P (Ge{P)
bondobservedforthemoleularmodel isprobably
re-latedtohargetransferfromphosphorustosilionand
germanium. With respet to the surfae normal, we
alulatedthe inlinationof theSi{P (Ge{P)bond as
=25.6 Æ
(30.5 Æ
)forthemoleularmodel,butonly13.5 Æ
(15.4 Æ
)forthedissoiatedmodel. Webelievethatthis
dierene arises from the dierene of the dimer tilt
anglesbetweenthemoleularanddissoiatedases. In
adition, we have found that the PH
3
as well as the
PH
2
{Si(Ge) speies preserve the trigonal harater of
thefreemoleule.
Table I. Strutural parameters for the moleular and
dissoiatedadsorptionmodelsofPH
3
onX(001){(21),
where X = Si or Ge. The symbols are explained in
Fig.1.
mole. disso.
bondleng.(
A) X=Si X=Ge X=Si X=Ge
X{X 2.38 2.57 2.39 2.54
X{P 2.35 2.49 2.27 2.38
X{H 1.51 1.57
P{H 1.45 1.46 1.46 1.46
bondang. ( Æ
)
X{X(!) 12.0 15.4 1.4 0.7
X{P() 25.6 30.5 13.5 26.1
X{H() 20.7 20.4
H{P{H 99.4 98.5 92.5 91.4
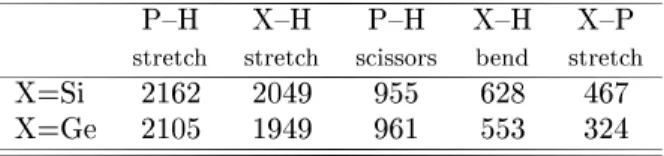
Foralulatingthezone-entre optialphonon
fre-quenymodesweset upa 3636eigenvalueproblem,
and only seleted modes that desribe a pronouned
surfaeharater. Theresultsforouralulated
zone-modesarepresentedinTable2. ThedereaseoftheX{
PstrethmodeasXhangesfromSitoPisonsistent
withthemasstrendgenerallyobserved. Thestrongest
experimentalindiationofthedissoiativeharaterof
theinteration ofphosphinewiththe silionsurfaeis
the detetion of Si{H streth mode imemediately
af-ter exposing Si(001) to PH
3
, as suggestedby Shan et
al [6℄. Ourrst-priniplesalulationsfurther support
this dissoiative adsorption model as we have
identi-edtheSi{Hstrethmodeat2049m 1
,inverygood
agreementwiththeexperimentalvaluesobtainedusing
HREELS[4℄(2100m 1
)andFTIS[6℄(2097m 1
).
Insummary,wehaveperformedrstpriniples
al-ulationsfortheatomistrutureanddynamial
prop-erties of the adsorption of PH
3
on the Si(001){(21)
and Ge(001){(21) surfaes. We nd that the
disso-iated state is energetially more favourable than the
moleular stateby1.70(0.81)eV, whereasthelatteris
0.58(0.25) eV more stable than the system omposed
ofthefreesilion(germanium)surfaeandPH
3
(g). We
havepresentedtheenergydiagramobtainedfora
pos-sible reationpathwayfrom the gas-phasePH
3 to the
moleularlyadsorbedstateandthentothedissoiated
state, and estimatethat theomplete dissoiation
o-ursattemperaturesaround110Kforsilionand240K
for germanium. The hemisorbed system is
hara-terisedbyelongatedSi{Si(Ge{Ge)dimersthatare
sym-metri in the dissoiative ase and asymmetri in the
moleular ase and by the fat that the Si(Ge){PH
2
as well as the PH
3
(ads) groups retain the pyramidal
geometry of thephosphine moleule. Our dissoiative
adsorptionmodelisfurthersupportedbyouralulated
vibrational modes, whih are in good agreementwith
available experimentalworks.
Table II. Zone enter vibratonal modes, in m 1
, for
the dissoiated adsorption models of PH
3
on X(001){
(21),where X=Si orGe.
P{H X{H P{H X{H X{P
streth streth sissors bend streth
X=Si 2162 2049 955 628 467
X=Ge 2105 1949 961 553 324
Aknowledgments
Referenes
[1℄ C.Li, S.John, E. Quinones, and S.Banerjee, J.Va.
Si.Tehnol.A14,170(1996).
[2℄ B.Tillak,ThinSolidFilms318,1(1998).
[3℄ Y.Wang,M.J.Bronikowski,andR.J.Hamers,J.Phys.
Chem.98,5966(1994).
[4℄ M.L.Colaianni,P.J.Chen,andJ.T.YatesJr.,J.Va.
Si.Tehnol.A12,2995 (1994).
[5℄ L. Kipp, R. D. Bringans, D. K. Biegelsen, J. E.
Northrup, A. Garia, and L.-E. Swartz, Phys.Rev. B
52,5843(1995).
[6℄ J.Shan, Y. Wang, and R. J.Hamers, J. Phys. Chem.
100,4961(1996).
[7℄ D.-S.Lin, T.-S.Ku, and R.-P.Chen,Phys.Rev.B61
2799(2000).
[8℄ R. Miotto, G. P. Srivastava, and A. C. Ferraz, Phys.
Rev.B63,125321 (2001).
[9℄ N.Troullier andJ.L. Martins, Phys.Rev.B43,1993
(1991).
[10℄ J.P.Perdew,K.Burke,andM.Ernzerhof,Phys.Rev.
Lett.77,3865(1996).
[11℄ M.J.PillingandP.W.Seakins,ReationKinetis