REVISTA
BRASILEIRA
DE
ANESTESIOLOGIA
PublicaçãoOficialdaSociedadeBrasileiradeAnestesiologiawww.sba.com.br
SCIENTIFIC
ARTICLE
Regulation
of
hypnosis
in
Propofol
anesthesia
administration
based
on
non-linear
control
strategy
Muhammad
Ilyas
a,
Ali
Khaqan
a,
Jamshed
Iqbal
b,∗,
Raja
Ali
Riaz
aaCOMSATSInstituteofInformationTechnology,DepartmentofElectricalEngineering,ChakShahzad,Pakistan
bNationalUniversityofComputerandEmergingSciences(FAST-NU),DepartmentofElectricalEngineering,Islamabad,Pakistan
Received28May2015;accepted17August2015 Availableonline11May2016
KEYWORDS
Closed-loop anesthesia;
Moderncontrol;
Biocontrol;
Pharmacodynamics; Pharmacokinetics
Abstract ContinuousadjustmentofPropofol inmanual deliveryofanesthesiafor conduct-ing a surgical procedure overburdens the workload of an anesthetist who is working in a multi-taskingscenario.Going beyond manual administrationandTargetControlledInfusion, closed-loopcontrolofPropofolinfusionhasthepotentialtoofferseveralbenefitsintermsof handlingperturbationsandreducingtheeffectofinter-patientvariability.Thispaperproposes aclosed-loopautomateddrugadministrationapproachtocontrolDepthOfHypnosisin anes-thesia.Incontrastwithmostoftheexistingresearchonanesthesiacontrolwhichmakesuseof linearcontrolstrategiesortheirimprovedvariants,thenoveltyofthepresentresearchliesin applyingrobustcontrolstrategyi.e.SlidingModeControltoaccuratelycontroldruginfusion. Basedonthederivedpatient’smodel,thedesignedcontrollerusesmeasurementsfromEEG toregulateDOHonBispectralIndexbycontrollinginfusionrateofPropofol.Theperformance ofthecontroller isinvestigatedandcharacterized withreal datasetof8patients undergo-ingsurgery.Resultsofthisinsilicostudyindicatethatforallthepatients,with0%overshoot observed,thesteadystateerrorliesinbetween±5.Clinically,thisimpliesthatinallthecases, withoutanyoverdose,thecontrollermaintainsthedesiredDOHlevelforsmoothconductionof surgicalprocedures.
©2016SociedadeBrasileiradeAnestesiologia.PublishedbyElsevierEditoraLtda.Thisisan openaccessarticleundertheCCBY-NC-NDlicense( http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗Correspondingauthor.
E-mail:jamshed.iqbal@nu.edu.pk(J.Iqbal).
http://dx.doi.org/10.1016/j.bjane.2015.08.011
0104-0014/©2016SociedadeBrasileiradeAnestesiologia.PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCC
PALAVRAS-CHAVE
Anestesiadecircuito fechado;
Controlemoderno;
Biocontrole; Farmacodinâmica; Farmacocinética
Controledahipnosenaadministrac¸ãodepropofolcombasenaestratégiadecontrole nãolinear
Resumo Oajustecontínuodepropofolnaadministrac¸ãomanualdeanestesiaparaarealizac¸ão deumprocedimentocirúrgicooneraacargadetrabalhodeanestesiologistasquetrabalhamem ambientemultitarefa.Indoalémdaadministrac¸ãomanualedainfusãoalvo-controlada(IAC),o controledecircuitofechadodainfusãodepropofoltemopotencialdeoferecerváriosbenefícios emtermosdemanejodasperturbac¸õesereduziroefeitodavariabilidadeinterpaciente.Este artigopropõeumaabordagemparaaadministrac¸ãoautomatizadadedrogasemcircuitofechado paracontrolaraprofundidadedahipnose(PDH)emanestesia.Emcontrastecomamaioriadas pesquisasexistentessobreocontroledaanestesiaqueusamestratégiasdecontrolelinearou de suasvariantesmelhoradas,anovidade dapresentepesquisaresidenaaplicac¸ãodeuma estratégiadecontrolerobusto;istoé,oControleporModosDeslizantes(CMD)paracontrolar comprecisão ainfusãodadroga.Combasenomodeloderivado dopaciente,ocontrolador projetadousaasmedic¸õesdoEEGpararegularaPDHnoBispectralIndex(BIS),controlandoa taxadeinfusãodepropofol.Odesempenhodocontroladoréinvestigadoecaracterizadocom umconjuntodedadosreaisdeoitopacientessubmetidosàcirurgia.Osresultadosdesteestudo insilicoindicamque,paratodosospacientes,com0%deexcessoobservado,oerrodeestado estacionário ficaentre±5. Clinicamente,issoimplicaqueemtodosos casos,semqualquer sobredosagem,ocontroladormantémoníveldesejadodePDHparaaconduc¸ãotranquilados procedimentoscirúrgicos.
©2016SociedadeBrasileiradeAnestesiologia.PublicadoporElsevierEditoraLtda.Este ´eum artigo OpenAccess sobumalicenc¸aCCBY-NC-ND( http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Thankstotechnologicaladvancements,thebenefitsoffered bymodernmedicinehave totallytransformedtheconcept of clinical surgery. Nowadays, surgical procedures can be performed with much ease and comfort. This incredible milestone has been achieved only through the research outcomesin modern anesthesia. Prior tothe discovery of anesthesia, surgery has to be conducted extremely fast. Historically, trivial techniques like application of cold, compression of nerve or reduction in cerebral perfusion wereemployedtokeeppatientunconscious.1Undoubtedly,
invention of inhalation gases in 1840 by Hickman was a pivotal steptowarddiscovery of anesthesiatofinally per-mitconductionofinvasivesurgeries.Thefirstprocedureof anesthesia,basedondiethylether,wasperformed in1842 byC.W.Long.Thisnewrevolutionaryconceptwaslateron termedasanesthesiameaninglackofesthesiai.e.sense.
Anesthesia is intensively used particularly in medical domain in many applications including surgical operation withincision,dentalsurgery andintensivecare.2The
pri-maryobjectiveofanesthesiaistoofferpainlessfeelingsto a patientunder operation bydriving him/her into uncon-sciousstatewithoutmemory.Theoverallfunctionalscenario ofanesthesiacanbecategorizedintothreetemporalphase insequence:induction,maintenanceandemergence. Dur-ing the firstphase, the objectiveis tobring apatient to areferenceDepthof Hypnosis(DOH). Itis thennecessary toadministerthe anestheticdrugin ordertomaintainan adequateDOH.Forinductionandmaintenanceof anesthe-sia,commonly usedintravenouslyadministeredanesthetic drugisPropofol.3Duringemergence phaseinpost-surgery
activities,vaporizerandother infusion devicesareturned offsoastoenablepatientstoawakefast.
During general anesthesia, Propofol is usually used togetherwithfastactingopioidse.g.remifentaniltohave asynergisticeffect.4Under-dosingofanestheticdrugsmay
leadto insufficient analgesia or awareness. On the other hand,itisdangerousforpatientstohaveexcessiveamount ofdrug.Thuscarefulmanagementoftheintravenousdrug delivery is the key factor behind successful anesthesia practice.It is desirous to accessthe depth of anesthesia togetherwithautomaticandinteractivedrugadministration withlittlehumaninterventionsoastoadjustdrugdosage accordinglyfor balancingtheanesthetic state, autonomic functionandresponsetonoxiousstimuli.
The procedures to administer intravenous drug deliv-ery have been evolved from simple manual delivery and computer-assisted automated Target Controlled Infusion (TCI)tomoresophisticatedClosed-LoopANesthesia(CLAN). Traditionally, hypnotic drug delivery rates in intravenous anesthesiaaremanuallycontrolledbyananesthetist.Doses are principally decided based on patient demographics, qualitatively measured signs (e.g. presence of certain reflexes, movement) and quantitatively measured signals (e.g. oxygen saturation, blood pressure, heart rate). The dosage scheme is then tuned by hit and trial to opti-mizeanesthesiaand toevade toxicity.TCI,alsoknownas Computer Assisted Continuous Infusion (CACI),5 relies on
population-based pharmacokinetic (PK) and pharmacody-namic (PD) models6 for calculating an adequate infusion
Awake state (100-90)
Light hypnotic
state
Moderate hypnotic state
Start deep hypnotic
state Flat line EEG Increasing
burst suppression
0 20
40 60
Surgical procedure band
80 100
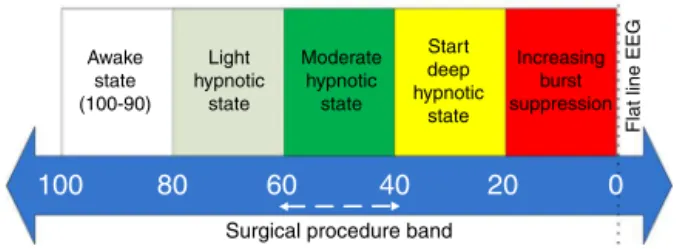
Figure1 BISscalingbandtoindicateDOHlevel.
of plasma concentration. This prediction is then used to trackthereferenceconcentrationthusdevisinganopenloop control paradigm. Instead of adjusting the infusion rate, the anesthetist manipulates the referenceconcentration, both reactivelyand proactively,usingcommercially avail-ableinfusionpumps.TCIsystemssufferfromdrawbacksof sensitivitytomodelnon-linearities anddisturbances since thereisnofeedbackofmeasurementondrugeffect.These drawbacks can be addressed by closing the control loop through DOH measurement, which is given by EEG-based monitors e.g. Bispectral Index (BIS).7 The value of BIS is
mappedto theDOH level of apatient basedon the scal-ingbandshowninFig.1.Thevalueof100---90corresponds to a fully awake state while level of 90---60 and 60---40 indicatelight and moderate hypnosis levelsrespectively.8
Themoderatelevelrepresentsthesurgicalprocedureband in which general surgery is performed by clinical profes-sionals.Levelbeyonddeephypnoticstate (40---20)isquite dangerous.9
InaCLAN system,drugeffectismeasured inrealtime andiscomparedwiththereferenceDOHtoobtainanerror signal.Basedonwhich,thesystem subsequentlyadjustsa druginfusionrate.ACLANsystemoffersseveralbenefitsin comparisonwithaTCIsystemincludingautomatichandling ofperturbations,precisecontrolofdruginfusionrate, min-imizing the effectof patientvariability and reducing the needofanesthetistintervention.
TrendtorealizeaCLANsystemhasbeenbasedontrivial orlinearcontrolapproaches.10Dong2proposedaCLAN
sys-temfortotalintravenousanesthesiabasedonProportional, Integral, Derivative (PID) controller. With BIS as sensory feedbackand Digital Signal Processing (DSP) based super-visorysystem,therealizedsystemwastestedon21healthy volunteersand 15patients undergoingsurgery. Exceptfor the2patients,satisfactoryclinical resultswereobtained. Anotherstudy11 basedonPIDcontrolinvestigatedthe
con-trolperformancewith10patientsundergoingelectivehipor kneesurgery. Themedianabsoluteperformance errorwas found tobe8%. The control strategywasable toprovide adequateanesthesiain9patientswithoscillatoryresponse recorded in BIS values for 3 patients. Other prominent studiesreportingPIDcontrolofanesthesiainclude.12,13
Com-paringconventionalPIDwithLinearModelPredictiveControl (LMPC),itisreportedinRef.14thatthelaterapproach out-performsinterms of robustnesstointraand inter-patient dynamicsandhandlingdisturbances,constraints and mea-surementnoise.Recent studies15---18 aim toimprovelinear
approaches by properly tuning the controllers to achieve sufficientrobustnessmarginsforidentifiableuncertainties. However,forcontrollawsbasedonthelinearapproaches, themodelofapatient,exhibitinganon-linearbehavior,is
linearized.Suchapproximationachievesgoodcontrol per-formance only if the difference between the predicted and actual closed-loop systems is small for the designed controller.19 The traditional PID controller cannot handle
disturbances like blood pressure changes, neural muscu-lar blockade and heart rate variability10 and may result
in oscillatorybehavior during clinicaltrials. Alsofor wide acceptanceofaCLAN systembyclinical professionalsand regulatory bodies, guarantees of robuststability and per-formance are must. Employing a non-linear and robust control strategy is therefore need of the hour in clinical anesthesia.
This research is aimed at unleashing the potential of a sophisticated control strategy i.e. Sliding ModeControl (SMC) to manage Propofol anesthesia infusion rate. The paper is organized as follows: Section II derives patient model. Section III explains the design details of SMC while Section IVpresentsresults basedonclinical param-eters of actual patients. Finally Section V comments on conclusion.
Patient
model
Thedynamicsofthehypnoticdrugiscategorizedinits phar-macokinetics(PK)andpharmacodynamics(PD)parameters. The PK parameter is used to govern the behavior of the infused drug in the body over time includingits distribu-tion,metabolism,absorptionandclearance20whilethePD
parameter representsthe drugconcentrationintheblood andthecorrespondingimpactcausedattheeffectsite.21
Onthebasis ofblood flow indifferentorgans,medical literaturedivideshumanbodyintovariouscompartments.22
Compartmentalmodelrepresentsabasickineticapproach todescribedrugabsorption,distributionandelimination.23
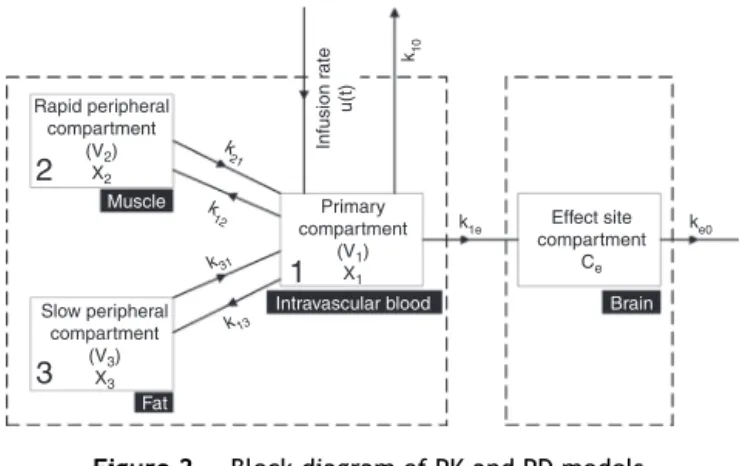
Relating plasma druglevelstoPD parameters,this model is intensively used in variousbiomedical and biotechnical applicationsbecauseoftheirinherentflexibilityand simplic-ity. The integratedPKPD structurefollowscompartmental modeling. In the present study, a three compartment PK model with an additional effect compartment has been adoptedowingtoitssufficientprecisionandcomputational efficiency.24Centredonaprimarycompartment
(intravascu-larblood)withvolumeV1,arapidperipheralcompartment (muscle)andaslowperipheralcompartment(fat),with vol-umesV2andV3respectively,areconnectedtotheprimary compartment.Thusdistributionandeliminationofthedrug betweenprimaryandperipheralcompartmentstakeplace withweightedrate constantsk12,k21,k13,k31 asdepicted inFig.2.Atanytime,thechangeinconcentrationofdrug in primary compartment is related tothe drug moved to andfromtherapidandslowperipheralcompartments.The induction and clearance of the drug takes place through the primary compartment. The drug eliminates from this compartmentinanexponentialfashion.17Attheeffectside
(brain),theconcentrationofthedrugismeasuredthrough thecorticalactivityinthebrainmeasuredthroughthe mod-ified form of EEGsignal.25 The extracted information can
thenbemappedtoDepthOfHypnosis(DOH)soastoanalyze patient’ssuitabilityforsurgicalprocedures.
2
Rapid peripheral compartment
(V2)
X2
Muscle
Fat
Intravascular blood Brain Slow peripheral
compartment
(V3)
X3
Primary compartment
(V1)
X1
Effect site compartment
Ce
k1e
k10
Infusion r
ate
u(t)
k
21
k
12
k31
k13
ke0
3
1
Figure2 BlockdiagramofPKandPDmodels.
ToderivethePKmodel,stateequationscorrespondingto thethreecompartmentscanbewrittenas(1)---(3)
˙
x1(t)=−k10x1(t)−k12x1(t)−k13x1(t)+k21x2(t)
+k31x3(t)+u(t) (1)
˙
x2(t)=−k12x1(t)−k21x2(t) (2)
˙
x3(t)=−k13x1(t)−k31x3(t) (3)
Laplacetransformof(1)---(3)yields(4)---(6)
sX1(s)=−(k10+K12+K13)X1(s)+k21X2(s)+k31X13(s)+(t)
(4)
sX2(s)=k12X1(s)−k21X2(s) (5)
sX23(s)=k13X1(s)−k31X3(s) (6)
Solving (4)---(6), the input---output relationship can be writtenas(7)
Dp(s)= X1(s)
U(s) =
(s2+s(k
21+k31)+k21k31)
(s3+s2(k
10+k12+k21+k13+k31)+s(k10k21+k10k31+k13k21+k31k21)+(k10k21k31))
(7)
where Dp(s) is the rate of drug absorption/metabolism withinthebodydefinedasdisposition rate.Rewriting(7), thegeneralformofPKmodelisobtainedas
Dp(s)= X1(s)
U(s) =
b2s2+b1s+b0 a3s3+a2s2+a1s+a0
(8)
where b2=1, b1=21+31, b0=2131, a3=1, a2=(10+12+13+31), a1=1021+1031+1231+ 1321+3121,a0=102131
The PD model indicating level of consciousnessrelates concentration of the drug in plasma to the effect site concentration andcan be derived based onthe state Eq.
(9)
˙
xe(t)=k1ex1(t)−ke0xe(t) (9)
ApplyingLaplacetransformon(9)
sXe(s)=k1eX1(s)−ke0Xe(s) (10)
Table1 Nomenclature.
Symbol Unit Name
u(t) mgseg−1 Infusionrate
k10 seg−1 Eliminationrateconstant
x1 mg Amountofdruginprimary
compartment
x2 mg Amountofdruginrapid
peripheralcompartment
x3 mg Amountofdruginslow
peripheralcompartment
xe mg Flowofhypnoticagentin
effectsite
k1e s−1 Rateconstantateffectsite
ke0 seg−1 Eliminationrateconstantat
effectsite
Ce mgL−1 Effectsiteconcentration
E0 --- Awakestage(100---90)
Emax --- Maximumeffectachieved
bydruginfusion
C50 mgL−1 Drugconcentrationathalf
ofthemaximumeffect
--- Sigmoidcurveslope
parameter
Consideringk1eandke0areequalbecauseofitsnegligible
volumeoftheeffectsitecompartment,thedispositionrate attheeffectsideisgivenby(11)
De(s)= Xe(s)
X1(s)
= ke0
(s+ke0)
(11)
BasedonthecascadednatureofPKandPDmodels,the overallpatientmodelcanfinallybewrittenas
Hp(s)= ke0 (s+Xe0)
∗ b2s
2+b 1s+b0 a3s3+a2s2+a1s+a0
(12)
BISis relatedwithanestheticeffectsiteconcentration
Ce(t)y throughnonlinearsigmoidmodeli.e.
BIS(t)=E0−Emax∗
Ce(t) Ce(t)
+C50
(13)
whereCe(t)canbecomputedbyintegrating(14)
˙
Ce(t)=−0.1068x1+0.456Ce (14)
Control
design
Patient model
Sigmoid
PD PK
BIS
S M C
b2s2 + b
1s + b0 ke0 Xe(s)
s + ke0
a3s3 + a2s2 + a1s+a0 R(s)
Figure3 Blockdiagramofoverallclosed-loopsystem.
toachievethedesiredDOHduringsurgicalprocedures.The overallobjectiveinthecontroldesignistominimizesteady stateerror soastomaintainDOHlevel withinacceptable rangeforsurgery.
The control law is based on SMC, which is one of the mosteffectiverobustcontroltechniquesfor highly nonlin-earsystemsoperatinginuncertainenvironmentssubjected todisturbances.SMCinvolvesdefiningaslidingsurface typ-ically a linear hyper-surface. The fundamental concept26
behindSMCistodrivethesystemdynamicsfromanyinitial statetotheslidingsurface(i.e.reachingphase).The sys-temisthenmaintainedonthissurfaceforallfuturevalues oftime(slidingphase).ThemajorbenefitofferedbySMCis itslowsensitivitytoplantdisturbancesanduncertainties.27
To designSMC,considering the slidingsurfacegivenby
(15)
=a1x1+a2x2+a3x3+a4xe (15)
or
˙
=a1˙x1+a2˙x2+a3x˙3+a4˙xe (16)
wherea1,a2,a3,a4aretuningparametersofthecontroller. With a1=1, values of other parameters are chosen in a
waythat0becomesHurwitzmonicpolynomial.This condi-tion ensures reduction in order of the system which can be represented with n−1 states. Such a system demon-stratesinsensitivitytomatcheduncertainties.Substituting thestateequations,(16)canbere-writtenas,
˙
=a1[(−k10−k12−k13)x1(t)+k21x2(t)+k31x3(t)+u(t)]
+a2[k12x1(t)−k21x2(t)]+a3[k13x1(t)−k31x3(t)]
+a4[k1ex1(t)−ke0xe(t)] (17)
The overall control law (u) consistsof equivalentcontrol (ueq)anddiscontinuouscontrol(udisc)i.e.
u=ueq+udisc (18)
The equivalent control forces the system dynamics to move tothe slidingsurface and dependsonthe states of the systemand state parameters.Itmakes thederivative ofslidingmanifoldequal tozero andcanbecomputedby putting=0alongthesystemdynamics(17).Thus,
ueq=−[(−k10−k12−k13)x1(t)+k21x2(t)+k31x3(t)]
−a2[k12x1(t)−k21x2(t)]−a3[k13x1(t)−k31x3(t)]
−a4[k1e1(t)−ke0xe(t)] (19)
Presence of disturbances or uncertainties may lead
=/ 0.Discontinuouscontrolhandlessuchdisturbancesand dependsongainandsignumfunction,whichexhibits switch-ingbehavior.Thus,
udisc=−ksign() (20)
wherek ∈ Rn×nisthediscontinuitygainmatrix.
Mathemat-ically,
sign()=
1for>0
−1for<0
(21)
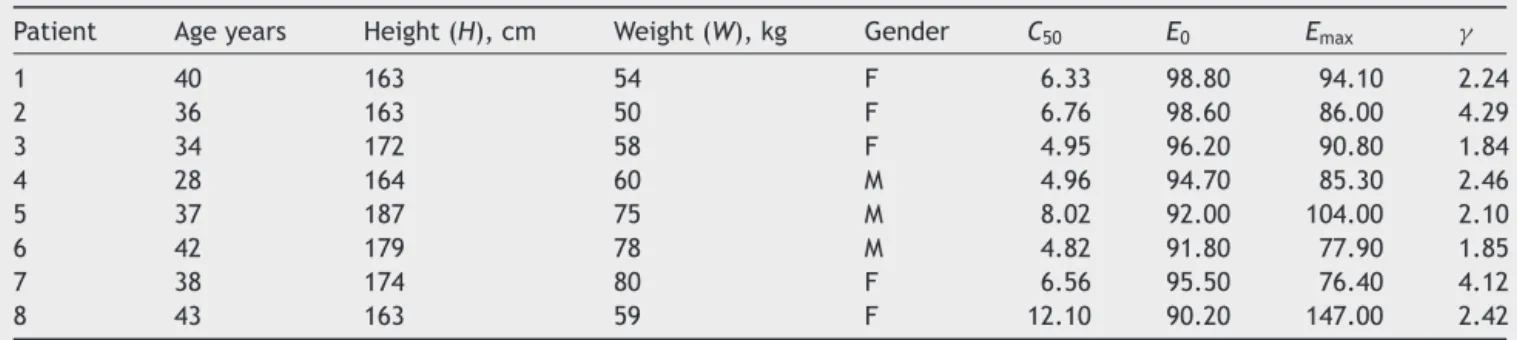
Toinvestigateandcharacterizetheperformanceofthe designed controller, clinical data including characteristic variablesofeightpatientsispresentedinTable2.8
Based on the patient’s attributes, clinical parameters computed using Schnider three compartmental model for Propofolaregivenbelow:
V1=4.27[l]
AV2=18.9−0.391(Age−53)[l]
AV3=238[l]
Table2 Clinicaldatasetshowingpatients’attributes.
Patient Ageyears Height(H),cm Weight(W),kg Gender C50 E0 Emax
1 40 163 54 F 6.33 98.80 94.10 2.24
2 36 163 50 F 6.76 98.60 86.00 4.29
3 34 172 58 F 4.95 96.20 90.80 1.84
4 28 164 60 M 4.96 94.70 85.30 2.46
5 37 187 75 M 8.02 92.00 104.00 2.10
6 42 179 78 M 4.82 91.80 77.90 1.85
7 38 174 80 F 6.56 95.50 76.40 4.12
a
b
0 50 100 150 200 250
–0.5 0 0.5 1
Time (sec.)
Plasma drug conc.
(mg/l)
Primary compartment Rapid peripheral compartment Slow peripheral compartment Effect site compartment
0 50 100 150 200 250
85 90 95 100
Time (sec.)
BIS
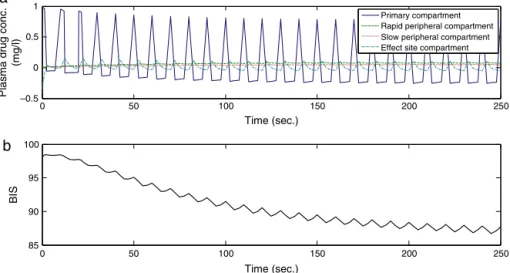
Figure4 Controller-lessadministrationofanestheticagentinpatient6:(a)drugconcentrationinvariouscompartmentsand(b) outputprofile.
Cl1=1.89+0.0456(W−77)−0.0681(LBM−59)
+0.0264(H−177)
Cl2=1.29−0.24(Age−53)
Cl3=0.836
whereLeanBodyMass(LBM)isafunctionofpatient’sgender, heightandweight.Formaleandfemale,itisrespectively givenas
LBM=1.1∗W−128∗W
2
H2
LBM=1.07∗W−148∗W
2
H2r 2
Therateconstantsk10,k12,k13,k21,k31dependonweight,
height,age,genderofthepatientandaregivenas:
k10=
Cl1
V1
k12=
Cl2
V1
k13=
Cl3
V1
b
a
c
0 50 100 150 200 250 1.4
1.6 1.8 2
Time (sec.)
Dr
ug
infus
ion
ra
te
(m
g/
s
)
0 50 100 150 200 250 –0.5
0 0.5 1 1.5 2
Time (sec.)
P
la
s
m
a
dr
ug
co
nc
.
(m
g
/l
)
Primary compartment Rapid peripheral compartment Slow peripheral compartment Effect site compartment
0 50 100 150 200 250 40
50 60 70 80 90 100
Time (sec.)
BI
S
a
b
c
d
0 50 100 150 200 250 –0.5
0 0.5 1 1.5 2
Time (sec.)
P
la
s
m
a
dr
ug
c
o
n
c
.
(m
g
/l
)
Primary compartment Rapid peripheral compartment Slow peripheral compartment Effect site compartment
0 50 100 150 200 250 –0.5
0 0.5 1 1.5 2
Time (sec.)
P
las
m
a
dr
ug
c
o
n
c
.
(m
g/
l)
Primary compartment Rapid peripheral compartment Slow peripheral compartment Effect site compartment
0 50 100 150 200 250 –0.5
0 0.5 1 1.5 2
Time (sec.)
Pl
a
s
m
a
drug
c
on
c.
(m
g
/l
)
Primary compartment Rapid peripheral compartment Slow peripheral compartment Effect site compartment
0 50 100 150 200 250 –1
0 1 2
Time (sec.)
Pl
a
s
m
a
d
rug
c
on
c.
(m
g
/l
)
Primary compartment Rapid peripheral compartment Slow peripheral compartment Effect site compartment
Figure6 Plasmadrugconcentrationin(a)patient4,(b)patient8,(c)patient2,and(d)patient7.
0 50 100 150 200 250
40 50 60 70 80 90 100
Time (sec.)
BIS (DOH)
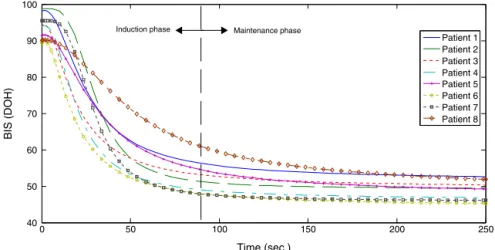
Patient 1 Patient 2 Patient 3 Patient 4 Patient 5 Patient 6 Patient 7 Patient 8
Maintenance phase Induction phase
k21=
Cl2
V2
k31=
Cl3
V3
Results
and
discussion
Thetrivialschemeofanestheticagentadministrationsimply consistsofacontroller-lessparadigm.Withsuchascheme,
Fig.4Apresentsplasmadrugconcentrationinvarious com-partmentswhileFig.4Bshowstheoutputprofileintheform ofBISsignal.BISvaluesarestillfarawayfromthedesired DOH level which is required for the general surgery. It is observed from theseresults that manipulatinganesthesia withoutadedicatedcontrollerintheloopcanbequiterisky andmaynotbesuitableinmanyoperationalscenarios.Using thisscheme,theaccuracyandprecisionofthedrug deliv-erytoapatientduringsurgeryistotallydependentonthe anesthesiologist experience. The critical response of this controllerbecomesmoreproblematicandcrucialespecially incaseofchildrenandcardiacpatients.
Employing a robust controllerin a closed-loop fashion completelychangestheresponse.Fig.5Apresentsthe con-trolleddruginfusionlevelusingSMCtechniqueforpatient6. PlasmadrugconcentrationinthecompartmentsofthePKPD structureisillustratedinFig.5B,wheretherateofchange ofdrug concentrationwithrespecttotimeinall thefour compartmentsofthebodyafterthedruginfusionisshown. Initiallythedrugconcentrationismaximumintheprimary compartment.Butasthedrugmovesbetweenprimaryand peripheral compartments, its level decays exponentially in the primary compartment and rises in the peripheral compartments.Thisflowofdruginthecompartmentsis rep-resentedthroughtheuseofrateconstants.TheoutputofBIS indicatorisplottedinFig.5C.Itclearlyshowsthatthe pres-enceofthecontrollerwithaclosed-loopfeedbacksystem dramatically improvesperformance ofanesthesiaprocess. TheoutputconvergestotherequiredlevelofBISforsurgery withinseconds.ThecontrollerthenmaintainsthisDOHlevel
–10 0 10 20 30 40 50 60
8 7 6 5 4 3 2 1
BI
S (
DOH
)
Patient no.
Reference Actual S/State error
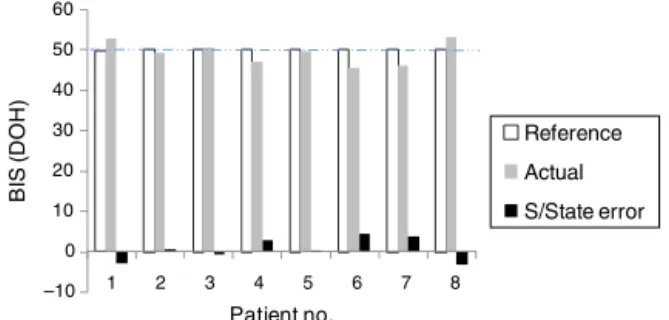
Figure8 SteadystateerrorshowingthatDOHisunderdesired range.
soastoassistanesthesiologist in ensuringsafer regionof operation.
Plasmadrugconcentrationinvariouscompartmentsisa functionoffactorsincludingpatients’age.Lessertheageof apatient,fasteristhemetabolism/eliminationofthedrug. Asanexample,comparethedrugconcentrationofpatients 4and8illustratedinFig.6AandBrespectively.Itisevident thatpatient 4 beingcomparatively youngerdemonstrates fastmetabolismofthedrugoccurringinprimary compart-mentthanpatient8.Comparisonofyoungandoldpatients disclosesthat the concentrationin rapid peripheral com-partment increases substantially due to the fast flow of Propofol from primary compartment. The same effect is reflectedinslowperipheralcompartmentandtheeffectsite compartment.
Incontrasttoage,theweightofapatientdoesnot sig-nificantlyaffecttheplasma drugconcentrationprofile.To investigatethiseffect,theconcentrationinpatients’2and 7(weight=30kg)hasbeencompared(Fig.6CandD).Itcan beseenthat theconcentrationofPropofol inthe primary compartmentofpatient2decaysatarelativelysamerate asthatofpatient7.Theminordifferenceintheresponses isduetodifferenceinagesofthepatients.Samefashionis observedregardingflowofdrugtoothercompartments.
Thedesignedcontrollerwiththederivedpatient’smodel is then simulated as per the dataset (Table 2). Simula-tionresults shown in Fig. 7 present the hypnosis level of 8 patients after the infusion of drug for surgery. These
0 50 100 150 200 250
1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9
Time (sec.)
Dr
ug
infu
si
o
n
ra
te
(m
g/
s)
Patient 1 Patient 2 Patient 3 Patient 4 Patient 5 Patient 6 Patient 7 Patient 8
responses indicate both the induction and maintenance phases of anesthesia. Initially, during induction phase, patientisin awakestate(DOHlevelnear100)andthenit entersintomoderatehypnoticstate(DOHlevelof40---60). Thislevelismaintainedfor safeexecutionofsurgical pro-cedures.In thisstudy, allthe patients achievedthe ideal hypnosislevel.However,forthesakeofquantification,Fig.8
shows steady state error considering DOH level of 50 as reference.Theerrorboundedinbetween±5isstillwithin acceptablerangeforsurgicaloperations.
The designed SMC provides different rates of Propofol infusioncorrespondingtodifferentpatients(Fig.9)dueto differenceinpatients’parameterslikeage,weight,height, genderandLBMtomaintainthedesiredlevelofDOH.The controllerinitiallypermitsinjectionoflargeamountofdrug tobringthe patientin unconsciousness state in induction phase of anesthesia. Once the desired hypnosis level is achieved,thenthecontrollerstrictlymaintainsthespecific infusionratethroughoutthemaintenancephaseof anesthe-siaforeachpatient.
Conclusions
ThispaperproposesSMC-basedlawfor adequateandsafe deliveryofPropofolanesthesiaforachievingdesired hypno-sislevels.Simulationresultsbasedonthedatasetcomprising of8realpatientswithdifferentclinicalparametersclearly witnessefficacyofthepresentedapproach.Withthehelpof medicalprofessionalsat National InstituteofHealth(NIH) Pakistan,we are goingto test the proposed CLAN in real surgicalscenarioaftermeetingmedicalsafetystandards.It isimperativetodemonstratepracticalbenefitsofCLANto convinceclinicians.TheCLANtechnique,thoughpotentially theeventualgoalofanestheticdruginfusionisstillinearly stagesofresearch.ItisanticipatedthatsuchaCLANsystem basedonanon-linearandrobustcontrolwillreplacemanual administrationaswellasTCIsysteminverynearfuture.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.BibianS[PhDThesis]AutomationinClinicalAnesthesia.Canada: UniversityofBritishColumbia;2006.
2.DongC[PhDThesis]ClosedLoopControlledTotalIntravenous Anaesthesia.UK:UniversityofPlymouth;2003.
3.WarpechowskiP,SantosATL,PereiraPJI,etal.Effectsof propo-fol on the cardiac conduction system. Rev Bras Anestesiol. 2010;60:441---4.
4.Absalom AR, Sutcliffe N, Kenny GNC. Closed-loop control of anesthesia using bispectral index: performance assess-mentinpatientsundergoing majororthopedicsurgeryunder combined general and regional anesthesia. Anesthesiology. 2002;96:67---73.
5.LiuN,ChazotT,GentyA,etal.Titrationofpropofolfor anes-thetic induction and maintenance guided by the bispectral index:closed-loopversusmanualcontrol:aprospective, ran-domized,multicenterstudy.Anesthesiology.2006;104:686---95.
6.SimoniRF,MiziaraLEDPG,EstevesLO,etal.Pharmacodynamic evaluationandphysical/chemicalanalysisoftwoformulations
ofpropofolusedintarget-controlledinfusion.RevBras Aneste-siol.2013;63:66---72.
7.NunesRR, Chave IMM,Alencar JCGD,etal. Bispectral index andotherprocessedparametersofelectroencephalogram:an update.RevBrasAnestesiol.2012;62:111---7.
8.Ionescu CM, De Keyser R, Torrico BC, et al. Robust predic-tivecontrol strategyappliedfor propofoldosingusing BISas acontrolledvariableduringanesthesia.IEEETransBiomedEng. 2008;55:2161---70.
9.Struys M,Desmet T, GreenwaldS,et al. Performance eval-uation of two published closed-loop control systems using bispectralindexmonitoring:asimulationstudy.Anesthesiology. 2004;100:640---7.
10.LanJY,AbbodMF,YehRG,etal.Review:intelligentmodeling andcontrolinanesthesia.JMedBiolEng.2012;32:293---307.
11.Absalom AR, Struys MMRF. An overview of target controlled infusionsandtotalintravenousanaesthesia.SanDiego,CA,USA: AcademiaPress;2007.
12.SolteszK,HeusdenK,DumontGA,etal.Closed-loop anesthe-siainchildren using aPID controller: a pilotstudy.In: IFAC conferenceonadvancesinPIDcontrol.2012.
13.SakaiT, MatsukiA, WhitePF,etal. UseofanEEG-bispectral closed-loop deliverysystem for administering propofol.Acta AnaesthesiolScand.2000;44:1007---10.
14.IngoleDD,SonawaneDN,NaikVV,etal.Linearmodelpredictive controllerforclosed-loopcontrolofintravenousanesthesiawith timedelay.ACEEEIntJControlSystInstrum.2013;4:8---15.
15.ZhusubaliyevTZ,MedvedevA,SilvaMM.Bifurcationanalysisof PID-controlledneuromuscularblockadeinclosed-loop anesthe-sia.JProcessControl.2015;25:152---63.
16.Heusden KV, Dumont GA, Soltesz K, et al. Design and clinical evaluation of robust PID control of propofol anes-thesia inchildren. IEEETransControl Syst Technol.2014;22: 491---501.
17.Soltesz K,Dumont GA,Ansermino JM.Assessingcontrol per-formance in closed-loop anesthesia. In: 21st Mediterranean conferenceoncontrolandautomation.2013.p.191---6.
18.WestN,DumontGA,HeusdenKV,etal.Robustclosed-loop con-trol ofinduction and maintenance of propofol anesthesia in children.PediatrAnesth.2013;23:712---9.
19.AjwadSA,IqbalJ,UllahMI,etal.Asystematicreviewofcurrent andemergentmanipulatorcontrolapproaches.FrontMechEng. 2015;10:198---210.
20.Shargel L, Wu-PongS, Yu AB. Applied biopharmaceuticsand pharmacokinetics.NY,USA:McGraw-Hill;2007.
21.HeusdenKV,SolteszK,KhosraviS,etal.Quantificationofthe variabilityinresponsetopropofoladministrationinchildren. IEEETransBiomedEng.2013;60:2521---9.
22.CoppensMJ,EleveldDJ,ProostJH,etal.Anevaluationofusing populationpharmacokineticmodelstoestimate pharmacody-namicparametersforpropofolandbispectralindexinchildren. Anesthesiology.2011;115:83---93.
23.BibianS, RiesCR, Huzmezan M,et al.Introduction to auto-mated drug delivery in clinical anesthesia. Eur J Control. 2005;11:535---57.
24.Schnider TW, Minto CF, Cambus P, et al. The influence of methodofadministrationandcovariatesonthe pharmacoki-neticsofpropofolinadultvolunteers.Anesthesiology.1998;88: 1170---82.
25.Hendrickx JF [PhD Thesis] The Pharmacokinetics of Inhaled AnestheticsandCarrierGases.Belgium:GhentUniversity;2004.
26.IslamRU,IqbalJ,KhanQ.Designandcomparisonoftwocontrol strategiesformulti-DOFarticulatedroboticarmmanipulator. ControlEngApplInform.2014;16:28---39.