REVISTA
BRASILEIRA
DE
ANESTESIOLOGIA
PublicaçãoOficialdaSociedadeBrasileiradeAnestesiologia www.sba.com.brSPECIAL
ARTICLE
Brazilian
Consensus
on
perioperative
hemodynamic
therapy
goal
guided
in
patients
undergoing
noncardiac
surgery:
fluid
management
strategy
---
produced
by
the
São
Paulo
State
Society
of
Anesthesiology
(Sociedade
de
Anestesiologia
do
Estado
de
São
Paulo
---
SAESP)
Consenso
Brasileiro
sobre
terapia
hemodinâmica
perioperatória
guiada
por
objetivos
em
pacientes
submetidos
a
cirurgias
não
cardíacas:
estratégia
de
gerenciamento
de
fluidos
---
produzido
pela
Sociedade
de
Anestesiologia
do
Estado
de
São
Paulo
(SAESP)
Enis
Donizetti
Silva
a,b,c,
Albert
Carl
Perrino
d,
Alexandre
Teruya
e,f,g,
Bobbie
Jean
Sweitzer
h,
Chiara
Scaglioni
Tessmer
Gatto
i,
Claudia
Marquez
Simões
a,b,j,
Ederlon
Alves
Carvalho
Rezende
k,
Filomena
Regina
Barbosa
Gomes
Galas
j,
Francisco
Ricardo
Lobo
l,m,
João
Manoel
da
Silva
Junior
k,
Leandro
Ultino
Taniguchi
n,o,
Luciano
Cesar
Pontes
de
Azevedo
a,o,p,
Ludhmila
Abrahão
Hajjar
a,i,j,
Luiz
Antônio
Mondadori
q,
Marcelo
Gama
de
Abreu
r,
Marcelo
Vaz
Perez
s,t,
Regina
El
Dib
u,
Paulo
do
Nascimento
Junior
u,
Roseny
dos
Reis
Rodrigues
f,p,
Suzana
Margareth
Lobo
l,m,v,
Rogean
Rodrigues
Nunes
c,w,x,
Murillo
Santucci
Cesar
de
Assunc
¸ão
f,∗aHospitalSírioLibanês,SãoPaulo,SP,Brazil
bSociedadedeAnestesiologiadoEstadodeSãoPaulo(SAESP),SãoPaulo,SP,Brazil cSociedadeBrasileiradeAnestesiologia(SBA),RiodeJaneiro,RJ,Brazil
dYaleUniversity,SchoolofMedicine,NewHaven,UnitedStates
eHospitaldeTransplantesdoEstadodeSãoPauloEuryclidesdeJesusZerbini,SãoPaulo,SP,Brazil fHospitalIsraelitaAlbertEinstein,SãoPaulo,SP,Brazil
gHospitalMoriah,SãoPaulo,SP,Brazil
hUniversityofChicago,NorthwesternSchoolofMedicine,Chicago,UnitedStates
iInstitutodoCorac¸ãodoHospitaldasClínicasdaFaculdadedeMedicinadaUniversidadedeSãoPaulo(INCOR/HCFMUSP),São Paulo,SP,Brazil
jHospitaldasClínicasdaFaculdadedeMedicinadaUniversidadedeSãoPaulo(HCFMUSP),InstitutodoCâncerdoEstadodeSão Paulo(ICESP),SãoPaulo,SP,Brazil
∗Correspondingauthor.
E-mail:murilloassuncao@gmail.com(M.S.C.deAssunc¸ão). http://dx.doi.org/10.1016/j.bjane.2016.09.007
kHospitaldoServidorPúblicoEstadual(HSPE),SãoPaulo,SP,Brazil
lFaculdadedeMedicinadeSãoJosédoRioPreto(FAMERP),SãoJosédoRioPreto,SP,Brazil mHospitaldeBasedeSãoJosédoRioPreto,SãoJosédoRioPreto,SP,Brazil
nFaculdadedeMedicinadaUniversidadedeSãoPaulo(FMUSP),DisciplinadeEmergênciasClínicas,SãoPaulo,SP,Brazil oInstitutodeEnsinoePesquisadoHospitalSírioLibanês,SãoPaulo,SP,Brazil
pHospitaldasClínicasdaFaculdadedeMedicinadaUniversidadedeSãoPaulo(HCFMUSP),UnidadedeTerapiaIntensiva, SãoPaulo,SP,Brazil
qA.C.CamargoCancerCenter,SãoPaulo,SP,Brazil rUniversityHospitalCarlGustavCarus,Dresden,Germany
sFaculdadedeCiênciasMédicasdaSantaCasadeSãoPaulo,SãoPaulo,SP,Brazil tUniversidadeFederaldeSãoPaulo(UNIFESP),SãoPaulo,SP,Brazil
uUniversidadeEstadualPaulista‘‘JúliodeMesquitaFilho’’(UNESP),DepartamentodeAnestesiologia,SãoPaulo,SP,Brazil vAssociac¸ãodeMedicinaIntensivaBrasileira(AMIB),SãoPaulo,SP,Brazil
wHospitalGeraldeFortaleza,Fortaleza,CE,Brazil
xCentroUniversitárioChristus(UNICHRISTUS),FaculdadedeMedicina,Fortaleza,CE,Brazil
Availableonline1October2016
Introduction
Thedefinitionof thepatient’s physicalstatus andcurrent clinicalconditionandtheemergencysurgerythathewillbe undergoing,translatedaccording to theAmerican Society ofAnesthesiologists(ASA),helpsin thedefinitionbutdoes notdefinemortality-predictability.1Cardiac2andrenal3risk
assessmentscales havebeen used,amongother multifac-torialand multidisciplinary measures,4,5 but theytend to
have no specificity and sensitivity coefficients that give thecaregivertheactualpredictabilityofthepreoperative complicationsanddeath.6
Several retrospective, prospective and observational studiesthatattemptedtoassessperioperativemortality7,8
demonstratedtheimportanceof recognizingthepatient’s risk9---11 as an initial measure to establish protocols
and guidelines related to hemodynamic monitoring, fluid replacement(fluidandtransfusion)settinggoalsfor resus-citation(6D1),andmultimodalcare(ERAS/Hitproject),12---14
aswellasothermeasuresthatcouldfacilitateinteractions betweenmonitoringandinterventionandsupportdecision makingusingclinicalguidelines.15,16
Moreover,severalotherstudieshavesuggestedthatthis approachmaychangetheoutcomesandsignificantlyreduce morbidityandmortality,withsignificant humanandsocial benefitsinterpretedfromthepointofviewofcost-effective measures.9
It is estimated that about 240 million surgical proce-duresareperformedannuallyaroundtheworld,wherethe standardmortalityrateincountriesandareassuchasUSA, Europe,andBrazilforpatientsunder60yearsofage under-going elective surgery and without chronic and clinically significantchangesis0.4---0.6%.6,7,17Forpatientsatrisk
(clin-icalstatus, typeof surgery, or a combination of factors), themortalityrateinnoncardiacsurgerycanbegreaterthan 26%.6,7Failuretoidentifypatientsatrisk,lackof
periopera-tiveresources,andlackofintensivepostoperativecareare amongthefactorsexacerbatingthismortality.6,7,11
Periop-erativehemodynamicoptimizationhascontributed,among otherthings,toreducemorbidityandmortality.
Aretrospectivestudy performedintheUKshowedthat amongstandard surgicalpatientsandpatients atrisk, the lattergroupaccountedfor80%ofdeathsofsurgicalpatients and over 80% of total spending in the UK. In the same study,Pearse statedthat ifphysicians donot identifythe patientsatriskandthereforedonotofferacomprehensive standard of care, thiswillsignificantly increase morbidity and mortality in this population. Consequently, hemody-namic monitoringandfluidreplacement aremandatoryin patientsatrisk.
Thebase ofdecision makingis theprevious knowledge ofhigh-riskpatients,useofprotocolsguidedbythe hemo-dynamic monitoring of both macro- and microcirculation, andtissueperfusionevaluationandresuscitationprotocols withfluidsandtransfusionsbasedonorienteddecisions,18
particularlythoserelatedtofluidresponsiveness.9,16,18,19
Fluidtitrationaccordingtoahemodynamicgoalis essen-tialtoimproveperioperativeoutcomes.20Somestudieshave
reported better outcomes when established guidelines of ‘‘restrictive’’ or ‘‘limited’’ fluid therapy were compared with standard care for gastrointestinal surgeries21---23 and
in patients withpulmonary dysfunction.24,25 These studies
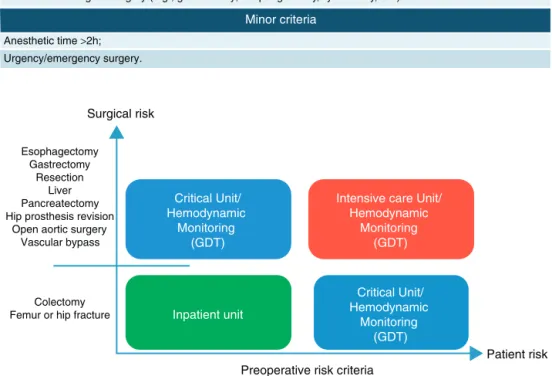
Preoperative risk criteria
Major criteria
Older than 70 years with some decompensated disease;
Previous severe cardiorespiratory disease (Coronary artery disease / COPD / Stroke);
Severe vascular disease involving large vessels;
Acute abdomen with hemodynamic instability;
Large blood loss (> 500 mL or > 7 mL.kg-1 younger than 12 years)
Septicemia;
Respiratory failure (need FiO2 >40% to keep Sat >90% or mechanical ventilation time >48h;
Renal failure;
Extensive oncological surgery (e.g., gastrectomy, esophagectomy, cystectomy, etc.)
Esophagectomy Gastrectomy
Resection Liver Pancreatectomy Hip prosthesis revision
Open aortic surgery Vascular bypass
Colectomy Femur or hip fracture Anesthetic time >2h;
Urgency/emergency surgery.
Minor criteria
Surgical risk
Critical Unit/ Hemodynamic
Monitoring (GDT)
Inpatient unit
Preoperative risk criteria
Patient risk Critical Unit/
Hemodynamic Monitoring
(GDT) Intensive care Unit/
Hemodynamic Monitoring
(GDT)
Figure1 Matrixforthedefinitionofhighriskpatients.35
limitationsrelatedtofunctionalhemodynamicparameters (FHPs), such as spontaneous breathing, non-standardized tidalvolume,non-standardizedairwaypressure/respiratory rate,non-sinusrhythm,neglecteddUp(deltaUP),andright heartfailure.26,27
PerioperativeGDTaimstoincreaseoxygendelivery(DO2)
duringmajorsurgeryapplyingatailoredhemodynamic moni-toringandtherapeuticinterventions.Whenperformedearly andintherightgroupofpatientswithadefinedprotocol,it hasshownthatGDTreducespostoperativemortalityinthe groupofhigherriskpatients16andmorbidityinallgroupsof
surgicalpatients.19,28
Thisguidelineevaluatedtheclinicalefficacyof hemody-namicGDTin reducingmorbidityandmortalityinsurgical patients,aswellasreducingthefinancialandhealthlosses associated withit. We have alsoproposed a set of surgi-calproceduresandpatientriskfactors(i.e.,age andASA) thatcouldbenefitGDT(Fig.1andTable1).Thus,theSão PauloStateSocietyofAnesthesiology(SAESP)invited anes-thesiologistsandintensivistsinvolvedinperioperativecare to establish a guideline for hemodynamic monitoring and fluidresuscitationinhigh-riskpatientsasacontributionto
healthprofessionalsandpolicymakersinvolvedinthecare ofpatientsatrisk.
Theuseofcriteriafordefiningpatientsatriskinthe pre-operativeperiodiscrucial.Afterreviewingseveralstudies andpublishedpapers,weconcludedthatthecreationofa table thatassociates high and low riskswith surgical risk wouldincreasethesensitivityandspecificityof character-izingthehigh-riskpatient.
Inthiscontext,werecommendusingthistableassociated withthiscarematrixanddecisionmaking.
Guidelinesandrecommendations
It has been shown in several meta-analysis that the use of protocols for perioperative hemodynamic support thatincreasestissueperfusionreducesorgandysfunctions, mortalityandhospitalization.29 Theseoutcomeswere
par-ticularlyevidentwhenappliedtomoreillpatients.16Akey
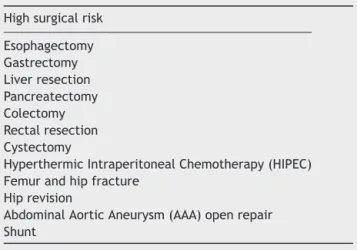
Table1 Surgicalproceduresto selectpatients who may
benefitfromGDT.
Highsurgicalrisk
Esophagectomy Gastrectomy
Liverresection
Pancreatectomy Colectomy
Rectalresection
Cystectomy
HyperthermicIntraperitonealChemotherapy(HIPEC)
Femurandhipfracture
Hiprevision
AbdominalAorticAneurysm(AAA)openrepair
Shunt
andcontractility)asaninteractionbetweentheautonomic responsetoanestheticagentsandvolumestatus.Thereis noglobal consensus on broad guidelines of fluid therapy, thus creatinglocal standardsas necessaries. Although we areofferingsomeguidance,thephysicianshoulddefinitely considercrucialaspectstoidentifyandtreatpatients, asso-ciatedwiththefollowingvariables:
(1) Patient status (health, age, physiology status, and comorbidities): these factors are some of the char-acteristics that may change the autonomic response and, consequently, hemodynamic parameters; there-fore, they are not necessarily related tofluids. Note that thisconsideration is mandatoryfor patients with conditionssuchasdiabetes,liverdysfunction,advanced atherosclerosis, and preoperative volume depletion. Moreover, we may not excludethe depth of anesthe-sia associated with peripheral chemoreceptors (e.g., neuromuscular blockade), baroreflex (e.g., opioid), impaired cardiaccontractility (e.g.,general anesthet-ics),orsympatholysis(e.g.,intravenousanesthetics).30
(2) Surgicalrisk(procedure(Fig.1andTable1),approach, andsurgicalexperience).
(3) Monitoringselection:theuseofstaticparameters(e.g., centralvenouspressureand/orpulmonaryartery pres-sure) has been associated with lower specificity and sensitivitycomparedtotheuseofthefluid responsive-nessdynamic parameters(functionalhemodynamics ---stroke volume variation [SVV], delta PP, etc.) aiming at maintain DO2 preoperatively. Forhigh-risk patients
undergoing medium or large surgery, the dynamic parametersassociatedwithGDT arerelatedtobetter outcomes.31
(4) Biomarkers for tissue perfusion adequacy (continuous monitoringoflactate,SvO2,ScvO2,CO2).
Methods
Searchstrategy
We searched the Cochrane Central Register of Controlled Trials(CENTRAL),CochraneLibrary(2015,Issue5),PubMed (1966 to May 2015), EMBASE (1980 to May 2015), Web
Table2 Searchstrategy.
((((hemodynamicgoal-directedtherapyORhaemodynamic
goal-directedtherapyORhemodynamicgoaldirected
therapyORhaemodynamicgoaldirectedtherapyOR
goal-directedtherapyORgoaldirectedtherapyOR
perioperativehemodynamicoptimizationOR
perioperativehaemodynamicoptimizationOR
goal-directedhemodynamicORgoal-directed
haemodynamicORgoaldirectedhemodynamicORgoal
directedhaemodynamicORoptimizationOR
haemodynamicORhemodynamicORhaemodynamicsOR
TGOGDTORhemodynamicsORgoal-directedfluid
therapyORgoal-directedfluidtherapiesORgoaldirected
fluidtherapyORgoaldirectedfluidtherapiesORfluid
therapyORfluidtherapiesORfluidchallengeORfluid
managementORperioperativehemodynamic
optimizationORperioperativehaemodynamic
optimizationORhemodynamicstabilizationOR
haemodynamicstabilizationORgoalorientedORgoal
targetedORcardiacoutputORcardiacoutputsOR
cardiacindexORoxygendeliveryORDO2ORoxygen
consumptionORoxygenconsumptionsORlactateOR
lactatesORsupranormal)AND(highrisksurgicalpatient
ORhighrisksurgicalpatientsORhigh-risksurgical
patientORhigh-risksurgicalpatientsORhighrisk
surgicalpatientORhighrisksurgicalpatientsOR
high-risksurgicalpatientORhigh-risksurgicalpatients))
AND(adultORadults))AND(humanORhumans))
of Science (1864 to May 2015), and Latin American and Caribbean Health Sciences (LILACS, 1982 to May 2015). Therewasnolanguagerestriction.Thedateoflastsearch wasMay13,2015.
Table1shows theelectronicdatabasesfromwhich the articleswere extracted andthe totalnumberof returned references.
As the search was conducted both by title and single words, it wasexpectedthat all GDT studieswith surgical patientswereidentified.
Table 2 shows the literature search strategy that was adaptedforeachelectronicdatabase.
Eligibilitycriteria
We consideredincludingonly randomizedcontrolledtrials (RCTs)orsemi-randomizedcontrolledtrials(semi-RCTs),all evaluatingadultpatients(>18years)undergoingnoncardiac surgeryandcomparingGDTwithstandardcare.
Semi-RCTs are those in which the treatment assign-mentwasobtainedbyalternation,useofalternatemedical records, alternate birth date or other alternate methods predictable.
We included studies that applied GDT at the follow-ingtimes:preoperatively,intraoperatively,and0---12hafter surgery.
The GDTinterventionsanalyzedintheseguidelinesare asfollows:
1. Fluidadministrationalone;and
2. Combinationoffluidandvasopressor/inotropes.
We consider any doses of the interventions described above.Thecontrolgroupreceivedstandard careora con-ventionalstrategyduringgoal-directedtherapy.
Weconsideredanalyzingthefollowingtypesofoutcomes: mortality;morbidities(e.g.,infections;cardiovascular, pul-monary,andrenalcomplications;anastomoticleak;nausea; vomiting);durationofhospitalstayanddurationof inten-sivecareunit(ICU)stay;durationofmechanicalventilation (calculated when the mean and standard deviation were reportedbytheauthors);andcosts(asanarrative descrip-tion).
Studyselection
Twoauthors independentlyselectedthepotentialstudies, assessedthetrialquality,andextracteddata.
Strengthofevidenceandsystemofclassification
andrecommendation
To create this guideline, the studies found in the liter-ature were classified according to the GRADE system of thestrengthof evidenceandstrengthof recommendation classification.32---34
Thegradingsystemclassifiesrecommendationsasstrong (Grade 1) or weak (Grade 2) according to the balance
betweenbenefits,risks,burdenandcosts,andthedegreeof confidenceinestimatesofbenefits,risks,andburden.The systemclassifiesqualityofevidence(asreflectedin confi-denceinestimatesofeffects)ashigh(GradeA),moderate (GradeB),orlow(GradeC)accordingtofactorsthatinclude theriskofbias,precisionofestimates, theconsistencyof theresults,andthedirectnessoftheevidence.32---34
To help readers, GRADE results of evidence were rep-resented using a color system in which green indicates strong recommendation (i.e.,1), red indicates weak rec-ommendation (i.e., 2), and yellow indicates studies with highprobabilityrecommendationbasedonevidences, but thisrecommendationwasdowngradedduetosomeproblem initsinternal and/or externalvalidity(Grade 1B/Cor 2, regardlessifA,B,orC).
Methodsusedforevidenceanalysis
AnevidencetablewasdevelopedforGDTbasedonacurrent literaturereviewandexpertpanelconsensus(Tables3---7). Wheneverpossible,wecalculatedtherelativerisk(RR)for mortalityandmorbidity,aswellasthemeandifference(MD) betweendurationofhospitalandICUstayanditsconfidence intervals(CI)of95%.Furthermore,thenumberofpatients whorequired treatment toprevent furtherpoor outcome (e.g.,thenumberofpatientsthatneededtobetreatedfor onetobenefitcomparedtoacontrolinaclinicaltrial)was calculatedtoobtainstatisticallysignificantresults.
Theaverageagecalculatedinthisstudywasbasedonthe averageageof bothgroups (i.e.,interventionandcontrol arms)ofeachstudyincludedinthisguideline.
Results
Tables3---7.
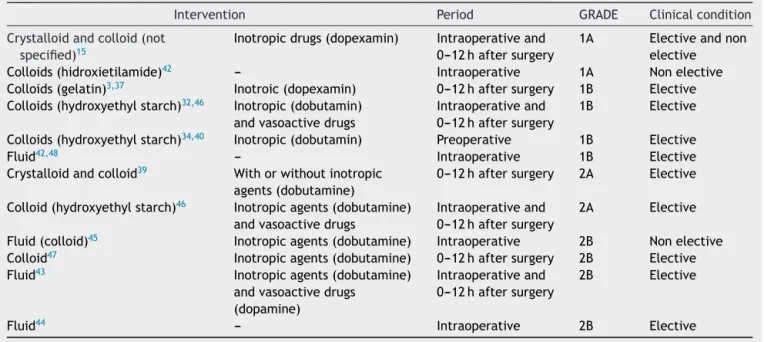
Table3 TreatmentoptionsforGDTinsurgicalpatientsaccordingtolevelofevidenceandgradeofrecommendation.
Intervention Period GRADE Clinicalcondition
Crystalloidandcolloid(not
specified)15
Inotropicdrugs(dopexamin) Intraoperativeand 0---12haftersurgery
1A Electiveandnon elective Colloids(hidroxietilamide)42 --- Intraoperative 1A Nonelective
Colloids(gelatin)3,37 Inotroic(dopexamin) 0---12haftersurgery 1B Elective
Colloids(hydroxyethylstarch)32,46 Inotropic(dobutamin)
andvasoactivedrugs
Intraoperativeand 0---12haftersurgery
1B Elective
Colloids(hydroxyethylstarch)34,40 Inotropic(dobutamin) Preoperative 1B Elective
Fluid42,48 --- Intraoperative 1B Elective
Crystalloidandcolloid39 Withorwithoutinotropic
agents(dobutamine)
0---12haftersurgery 2A Elective
Colloid(hydroxyethylstarch)46 Inotropicagents(dobutamine)
andvasoactivedrugs
Intraoperativeand 0---12haftersurgery
2A Elective
Fluid(colloid)45 Inotropicagents(dobutamine) Intraoperative 2B Nonelective
Colloid47 Inotropicagents(dobutamine) 0---12haftersurgery 2B Elective
Fluid43 Inotropicagents(dobutamine)
andvasoactivedrugs (dopamine)
Intraoperativeand 0---12haftersurgery
2B Elective
Fluid44 --- Intraoperative 2B Elective
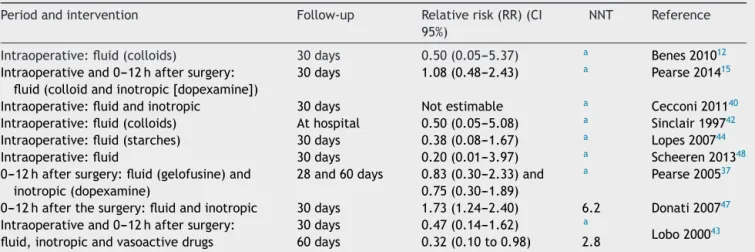
Table 4 Relative risk and the number needed to treat mortality in GDT surgical patients according to the degree of recommendation.
Periodandintervention Follow-up Relativerisk(RR)(CI
95%)
NNT Reference
Intraoperative:fluid(colloids) 30days 0.50(0.05---5.37) a Benes201012
Intraoperativeand0---12haftersurgery: fluid(colloidandinotropic[dopexamine])
30days 1.08(0.48---2.43) a Pearse201415
Intraoperative:fluidandinotropic 30days Notestimable a Cecconi201140
Intraoperative:fluid(colloids) Athospital 0.50(0.05---5.08) a Sinclair199742
Intraoperative:fluid(starches) 30days 0.38(0.08---1.67) a Lopes200744
Intraoperative:fluid 30days 0.20(0.01---3.97) a Scheeren201348
0---12haftersurgery:fluid(gelofusine)and inotropic(dopexamine)
28and60days 0.83(0.30---2.33)and 0.75(0.30---1.89)
a Pearse200537
0---12hafterthesurgery:fluidandinotropic 30days 1.73(1.24---2.40) 6.2 Donati200747
Intraoperativeand0---12haftersurgery: fluid,inotropicandvasoactivedrugs
30days 0.47(0.14---1.62) a
Lobo200043
60days 0.32(0.10to0.98) 2.8
aThenumberneededtotreat(NNT)wasnotcalculatedbecausetherewasnosignificantdifferencebetweengroups.Whenthereisno treatmenteffect,theabsoluteriskreductioniszeroandtheNNTisinfinite.
Studyselection
Weidentifiedatotalof12,165records,afterremoving dupli-cates,throughdatabasesearchesfororiginalreview(Fig.2). After sorting by title and, subsequently, by abstract, we foundfull copiesof 600recordsthatwerepotentially eli-gibleforinclusionintheguideline.Weexcluded584studies for the following reasons: off-topic; editorials or letters; narrativereviews;casestudy; cardiacstudies;duplicates; publishedprotocols;andcohort andcase---controlstudies. Therefore,15RCTsandsemi-RCTsmettheinclusioncriteria forthisguideline(Table8).
HowtoselectpatientswhomaybenefitfromGDT? GRADE:1C
Response: We recommend using GDT according to patient’s risk and surgical risk because these factors can improve the following outcomes: mortality, morbid-ity (e.g., infection; cardiovascular, pulmonary and renal complications;anastomoticleaks;nausea;vomiting);length of hospital and ICU stay; and duration of mechanical ventilation35 (Fig. 2). We recommend the use of GDT in
patientsagedover65yearsandASA≥IIandpatients under-going≥2hofsurgeryorwithexpectedbloodlossover500mL orurgent/emergencysurgeryoroneofthesurgical proce-dureslistedinTable1.36
IsGDTmoreeffectiveandsaferthanstandardcareto reducemortalityandmorbidityinhigh-risksurgical patients?
GRADE:1A
Response:Yes.TheuseofGDTreducesmorbidityin dif-ferentagegrouppatients,whileitreducesmortalityonlyin veryhigh-riskpatients.15,37---42
Arguments: Reduction in mortality with GDT in high-risk patients was seen in patients with early mortality rates>20%.41,43 This high mortalityrate is consistent with
mortalityratesofpatientsundergoinghigh-risksurgery, pre-viously reported in Brazil.43,44 The use of protocols with
# of records identified through database searching 13,791
# of additional records identified through other sources 0
# of records after duplicates removed 12,165
# of records screened 12,165
# of full–text articles assessed
for eligibility 600
# of studies included in this
guideline 14
# of records excluded 11,565
# of records excluded, and reasons 586
409 Off–topic
45 reviews
76 case report or letter for the editor
56 observational studies PubMed 5,394
EMBASE 7,128
CENTRAL 111
Web of Science 330
LILACS 828
Table5 TrendsforlowerandhighermorbidityinGDTsurgicalpatientsaccordingtothedegreeofrecommendation:relative
riskandnumberneededtoharmoccurrence.
Period Intervention Outcome Relativerisk(RR)(CI
95%)
NNT Reference
0---12hafter
surgery
Fluid(crystalloidand
colloid),andinotropic
drugs(withorwithout
dobutamine)
Morbiditydefinedin
POMS(postoperative
morbiditiesSurvey)
1.04(0.96---1.11) b Ackland201539
Pulmonary 1.11(0.96---1.29) Renal 1.03(0.91---1.17) Gastrointestinal 1.11(0.89---1.38) Cardiovascular 1.09(0.68---1.74) Hematologic 1.33(0.56---3.16) Pain 1.14(0.98---1.33)
Intraoperativeand 0---12hafter surgery
Fluid(colloidand inotropic(dopexamine)
Compoundoutcomes (postoperative mortalitywithin30 daysandpredefined seriouspostoperative complicationsa)
0.84(0.71---1.01) b Pearse201415
Fluid(colloidand inotropic(dopamine)
Ischemiaor
myocardialinfarction
1.24(0.50---3.11)
Cardiacorrespiratory arrest
1.14(0.56---2.29)
Gastrointestinal bleeding
1.62(0.68---3.85)
Fluid(crystalloid, colloid)andinotropic (dobutamine)
Complications 1.13(0.69---1.85) Bisgaard2013a,38
Intraoperativeand 0---12hafter surgery
Fluid(crystalloidsand colloids),andinotropic drugs(withorwithout dobutamine)
Infection 0.97(0.66---1.41) b Ackland201539
Neurologic 0.51(0.24---1.09) Wound 0.97(0.20---4.68)
Fluid(colloids)and inotropic(dobutamine)
Complications 0.99(0.26---3.78) b Donati200747
Cardiocirculatory failure
0.20(0.02---1.64)
Respiratoryfailure 0.99(0.26---3.78) Renalfailure 0.28(90.6---1.31) Liverfailure 0.25(0.05---1.12) Hematologicalfailure 0.14(0.01---2.67)
Fluid(gelofusine)and inotropic(dopamine)
Complications (infection, respiratory, cardiovascular, abdominal,and massive postoperative bleeding)
0.64(0.46---0.89) 4 Pearse200537
Fluidandinotropic Totalandmajor complications
Total:0.13(0.02---0.91) Important:0.80 (0.64---1.02)
2.8 Cecconi201140
Fluid,inotropicand vasoactivedrugs
Arrhythmia 0.14(0.01---2.46) b Lobo200043
Table5 (Continued)
Period Intervention Outcome Relativerisk(RR)(CI
95%)
NNT Reference
Intraoperative Fluid(starches) Respiratory
complications
0.38(0.23---0.95) 2.5 Lopes200744
Renalcomplications 0.09(0.01---0.59) 1.5 Arrhythmia 0.47(0.14---1.57)
b
Infection 0.47(0.21---1.08) Acutepulmonary
edema
0.19(0.01---3.66)
Abdominal 0.31(0.01---7.21)
Fluidandinotropic Survivorswith complications
0.80(0.54---1.19) b Bartha201345
Fluid(colloids) Seriouscomplications 0.32(0.15---0.69) 4 Benes201012
Complications 0.51(0.33---0.80) 3.6
Fluid Woundinfection 0.07(0.00---1.11) b Scheeren201348
Inotropic(dobutamine) andvasoactivedrugs
Complications 0.40(0.20---0.82) 2.22 Bisgaard2013b46
aPulmonaryembolism,ischemiaormyocardialinfarction,arrhythmia,cardiacorrespiratoryarrest,limborfingerischemia,cardiogenic pulmonaryedema,respiratoryacutestresssyndrome,gastrointestinalbleeding,intestinalinfarction,anastomosisbreakdown,paralytic ileus,acutepsychosis,stroke,acutekidneyinjury,infection(sourceuncertain),urinarytractinfection,surgicalsiteinfection,organor spaceinfection,bloodinfection,nosocomialpneumonia,andpostoperativebleeding.
b Thenumberneededtotreat(NNT)wasnotcalculatedbecausetherewasnosignificantdifferencebetweengroups.Whenthereisno treatmenteffect,theabsoluteriskreductioniszeroandtheNNTisinfinite.
supranormal physiological targets decreased morbidity in high-risk patients.16 A careful hemodynamic monitoring
before, during, and after surgery to adjust fluid therapy facilitates the recognition and early correction of tissue hypoperfusion. The reduction in complication rates was deeper in high-risk patients, protocols with supranormal physiologicaltargets, andcasesreceiving inotropicagents inadditiontofluid.Althoughtheuseofinotropicagentshas notbeen recommended in the study OPTIMISE, when the studywaslateraddedtothesystematicreviewand meta-analysisofthestudygroup,theinterventionwasassociated withareductionincomplicationrates.15
IsGDTeffectiveandsafewhenappliedintraoperatively toreducemortalityandmorbidityinhigh-risksurgical patients?
GRADE:1B
Response: Yes,GDT is safeandeffective when applied intraoperatively toreduce postoperative complications in high-risksurgicalpatients.12,15,38,40,42,43,45---48
Arguments: Several studies have suggested that GDT applieduponincreasedbloodflowmayreducepostoperative complications.Mostof thesestudies wereconducted dur-ingtheintraoperativeperiod.49---51 Allthesestudiesshared
theneed forenhancedmonitoring withagavage49,52
arte-rial catheter,43,50,51 or pulmonary artery catheter (PAC).12
These studies concluded that hemodynamic optimization duringsurgeryimproves thesurgicaloutcomesinhigh-risk patients, andall forms of monitoring appeartobe effec-tive.Goal-directedtherapytypicallyusesamonitoringtool forassessingcardiacfunctioncontinuouslyandviaasetof protocolinstructions,administrationoffluidandvasoactive agentsistitratedtooptimizecardiacperformance.Central tothesestudiesisthattheGDTshouldnotbedefinedbythe presenceorabsenceofamonitoringdevicebutbyexplicit treatmentobjectives,suchasmaintenanceofcardiacindex andbloodvolumedynamicparameters.Generally,inthese surgical patients, GDT should be provided during all pro-cedures, from induction to 6---24h in the ICU. Recently, Pearseetal.15 reportedtheOPTIMISEresults,apragmatic
multicenter trial performed in 17 hospitals with734 ran-domlychosenhigh-riskpatientsundergoinggastrointestinal surgery to receive standard care or GDT intraoperatively and for 6h after surgery. The intervention tested in this study consisted of a dopexamine infusion+bolus adminis-tration of colloid (250mL) to maintain maximum stroke volume (SV) during the study period.SV was determined usinganadvancedmonitor.Theprimaryendpointincidence
Table6 MeandifferenceinlengthofhospitalandICUstayinGDTsurgicalpatientsaccordingtothedegreeofrecommendation.
Intervention Typeofoutcome Meandifference(ND)(95%CI) Reference
Intraoperative:fluid ICU −12.00(−34.89---10.89) Scheeren201348
Table7 Interventioncharacteristicsoftheincludedstudiesbyperiodoftime. Electiveor
non-elective surgery
Surgerytype Meanage Intervention Monitor Type
Technique Maintarget Calibrated GRADE Reference
Preoperativeandintraoperative
Non-elective Orthopedic 74.7 Fluid(colloid) Doppler Minimally invasive
Systolicvolume; correctflowof time;CO
Yes 1B Sinclair
199742
Elective Totalhip
arthroplasty
66 Fluidand
inotropic Vigileo/ FloTrac system Minimally invasive
DO2>600mL/
min/m2
No 1A Cecconi
201140 Elective Retroperitoneal, aorticmajor opensurgery, majorrenal, bladdersurgery, and hysterectomy and oophorectomy forcancer
Noreport Fluid Notapplied Notapplied Notapplied Not
applied 2B Cuthbertson 201156 Intraoperatory Elective Abdominal importantand radical cystectomy
70.5 Fluid(colloid) Vigileo/
FloTrac system Minimally invasive Variationin strokevolume
No 1B Scheeren
201348
Elective Intra-abdominal 66.5 Fluid(colloid) Sistema
Vigileo/ FloTrac system Minimally invasive Variationin strokevolume Not reported
1B Benes201012
Non-elective Orthopedic 85.5 Fluid(colloid)
andinotropic (dobutamine)
Lidco Minimally
invasive
Oxygendelivery Yes 2B Bartha
201262
Elective Upperandlower
gastrointestinal, hepatobiliary, urological
62.5 Fluid(starch) Pulse
pressure variation
Minimally invasive
DeltaPP≤10% Not applied
2B Lopes200744
Intraoperativeand0---12haftersurgery
Both Upperandlower
gastrointestinal, smallintestine withorwithout pancreas, urologic, gynecologic.
71.7 Fluid(colloid)
andinotropic (dopamine)
Lidcorapid Minimally invasive
Systolicvolume No 2B Pearse
201415
Elective Aortic 68 Fluid
(hydroxyethyl starch)and inotropic (dopamine) and vasopressors
Lidcoplus Minimally invasive
DO2 Yes 2B Bisgaard
2013a38
Elective Upperandlower
gastrointestinal andvascular. 72.5 Fluids (crystalloids, colloids), inotropic (dobutamine) and vasopressors
Lidcoplus Minimally invasive
DO2 Yes 1B Bisgaard
2013b46 Elective Total esophagectomy, gastrectomy, pancreatectomy, bowelresection, abdominal aorticaneurysm 62.7 Fluid, inotropic (dobutamine) and vasopressors (dopamina) Pulmonary artery catheter
Invasive DO2 No 2B Lobo200043
0---12haftersurgery
Elective Vascular,upper andlower gastrointestinal, hepatobiliary, urological 61 Fluid (gelofusine) andinotropic (dopexamine)
Lidcoplus Minimally invasive
Oxygendelivery index;systolic volume
Yes 1B Pearse
Table7 (Continued) Electiveor
non-elective surgery
Surgerytype Meanage Intervention Monitor Type
Technique Maintarget Calibrated GRADE Reference
Elective Abdominal aorticaneurysm, intestinal resectionfor cancer, pancreaticoduo-denectomy, aortoiliacbypass
66 Fluid
(colloids)and inotropic (dobutamine)
Notapplied CVC O2Ext (SaO2−ScvO2/ SaO2)<27%
Not applied
1B Donati
200747
Elective Upper
gastrointestinal, liverand hepatobiliary resection,lower gastrointestinal andvascular
68 Fluid
(crystalloids andcolloids) andinotropic (dobutamine)
Lidcoplus Minimally invasive
Cardiacoutput Yes 2A Ackland
201539
(acompositeof pre specified postoperativecomplications within30daysofthesurgery)waslowerinGDTgroup(36.6% vs. 43.4% [relative risk (RR) 0.84; (95% CI, 0.71---1.01)]; absolute risk reduction, 6.8% [95% CI, −0.3% to 13.9%]). This reduction, consistent with the benefits observed in manyprevioustrials,12,38,40,42,43,44---48wasstillsignificantafter
adjustmentforinitialriskfactorsorafterdeletingthefirst 10patients.
The authors performed an additional analysis, includ-ingtheOPTIMISEresultsinanupdatedsystematicreview.15
Theseresults furtherstrengthenedthe generalconclusion thatGDTofsomesortislikelytobebeneficialtohigh-risk patientsandhasfewadverseeffectsdocumented.Findings ofameta-analysisof38trials,includingdatafromOPTIMISE studysuggestthattheinterventionisassociatedwithalower incidenceofcomplications(intervention,488/1548[31.5%] vs.control,614/1476[41.6%];RR,0.77[95%CI,0.71---0.83]) andnon-significant reductions in mortalitywithin 28 days and30days(intervention,159/3215deaths[4.9%]vs. con-trol,206/3160deaths[6.5%];RR,0.82[95%CI,0.67---1.01]) and mortality in the longer follow-up period (interven-tion,267/3215 deaths[8.3%]vs.control,327/3160deaths [10.3%];RR,0.86[95% CI,0.74---1.00]). Thesefindings are consistentwithreports fromCenters forMedicare & Med-icaidServices53 andNationalInstitutefor HealthandCare
Excellence,54whichrecommendedtheuseofhemodynamic
therapyalgorithms.
Table 8 Electronic databases, date of last search, and
numberofreturnedreferences.
Electronic databases
Dateoflast
search
Numberof
returned references
PubMed 1966toMay2015 5394
CENTRAL Issue05,2015 111
EMBASE 1980toMay2015 7128
WebofScience 1864toMay2015 330
LILACS 1982toMay2015 828
IsGDTeffectiveandsafewhenappliedpostoperatively toreducemortalityandmorbidityinhigh-risksurgical patients?
GRADE:1A
Response: Yes. We recommend applying GDT after surgeryinhigh-risksurgicalpatients.
Arguments: Studies have shown that this strategy may contribute toreduce morbidity15,37 GDT shouldbeapplied
in the first8h postoperativelyand requires hemodynamic monitoringtoguidefluidreplacement,inotropes, vasopres-sors,andvasodilators.Inacost-benefitanalysis,Ebmetal.55
reportedthatGDTcouldreducecostsby£2631.77/patient
and£2134.86/in-hospitalsurvival,indicatingthatitis
effec-tivebothclinicallyandintermsofcost.Additionalcostsof implementationcan beoffset bysavings fromcost reduc-tion due to reduction in complication rates and hospital stay.Inaddition,thisstudyshowedthatGDTnotonly pro-longedquality-adjustedlifeexpectancy(0.83yearsand9.8 months), but alsoled toa cost reductionprojection dur-ing life of £ 1285.77, resultingin a negativeincremental
cost-benefitrateof£1542.16/quality-adjustedlife-year.55
Shouldwehemodynamicallymonitorpatientstoapply GDTinhigh-risksurgicalpatients?
GRADE:1A15,40,43,44
Response: Yes, every patient who will undergo GDT shouldbehemodynamicallymonitored.Werecommendany monitorthatisavailabletoestimatethecardiacoutput(CO) or differenttoolsassociatedwithpulseoximeter (plethys-mograph variability index--- PVI), bedside monitors(pulse pressurevariation---PPV)andCOmonitors(strokevolume variation---SVV,SV,oxygensupply---DO2).Inaddition,other
toolshavebeenusedtoguideGDT,suchasPAC,esophageal Doppler, andmethods for pulse curve analysis.It is note-worthythatnoinvasivemonitoring,suchaspulseoximetry withplethysmographicanalysisormethodsassociatedwith leg elevation maneuvers should be used as a functional hemodynamicparameters(FHP).12,15,37---40,42,43,45---48,56,57
that‘‘(...)feedbackfromanesthesiologyproviderswasthat
thisprotocol [NICENHS protocol suggesting fluidto maxi-mizeSV]forcedthemtogivemorefluidsthanthoughtshould begiven,and teamleadersdecidedtoinclude thestroke volumevariation(SVV)asthetriggertofluidadministration toincreasephysicians’adherence.59
Arguments: All studies used some type of device to monitorhemodynamicparameters.ToapplyGDT,itis nec-essary first to establish a protocol for delivery of oxygen andpreventtissuehypoperfusion,andmanyprotocolshave been published in the literature. In general, fluid and inotropes are used. Fluid should be administered when patientsrequireincreasedperfusionandarealsoresponsive tovolume.60
Fluid responsiveness maybe assessed by PPV,SVV, PVI or by the superior vena cava compressibility. It is impor-tanttoadjustandmakesurethatpatients’parametersare eligiblefortheassessmentofthefluidresponsiveness varia-bles,withoutrespiratorytriggers,arrhythmiasoropenchest surgery,andtidalvolumeofatleast8mL/kgestimatedby height.Postoperatively,ifthepatientisbreathing sponta-neously,astrategy called ‘‘passiveelevationof thelegs’’ maybeusedasameanstochange theventricularpreload associatedwiththe measurementof the change instroke volume,whichprovidesanaccuratemeanstoguidethe ther-apyprovidingfluidratesinhigh-riskpatients. Patientsare consideredresponsiveifthecardiacoutputincreasesfrom 10% to15% ofbaseline values.When dynamic parameters (PPV,SVV,PVI)maynotbeused,aCOmonitorisrequired toquantifychangesinstrokevolumeorDO2.Animportant
aspecttobeavoidedwhileapplyingGDTisfluidoverload; thatis,whenpatientsdonotderivebenefitfromthefluid administration; otherwise, thereis no increase in cardiac output.
WhattoolsshouldbeusedforGDT? GRADE:1A
Response:Werecommendanymonitorthatisavailable toestimatethecardiacoutputordifferenttoolsassociated withpulseoximeter(PVI),bedsidemonitors(PPV),andCO monitors(SVV,SV,DO2).ToapplyGDTproperly,thedoctor
mustrelyonSVoptimizationbasedonDO2orPPV
optimiza-tion(thefirstrequiresaCOmonitor,butnotthelatter).In addition,othertoolshavebeenusedtoguideGDT,suchas PAC,esophagealDoppler,andmethodsofpulsecurve analy-sis.Therefore,allthesemethodsmaybeusedastheyhave beenassociatedwithreductionsinmorbidityand/orhospital stay.12,15,37---40,42,43,45---48,56
Arguments:Studieshavebeenbasedonprotocolsandnot onspecific devices;nomonitoringtechnique byitself can improve outcomes. Some devices offer more advantages, suchasbeinglessinvasiveorminimallyinvasive.For exam-ple, pulsecurve analysis, transpulmonary thermodilution, andesophagealDopplerfeatureparameterstoapplyGDT. However,thesemethodsaregenerallymoreexpensiveand arenotofferedbytheUnifiedHealthSystem(SUS)--- Min-istryofHealth.Inthisscenario,pulmonaryarterycatheters maybeusedtoreplaceminimallyinvasivetechniques. Mon-itoringrequirementsmayvarywithtimeanddependonthe localavailabilityofequipmentandtraining.Itisvery impor-tanttoemphasize thattheentireteamshouldbefamiliar
andtrainedtoinsertdevices,manageandinterpretdata, andapplystrategies.This recommendationis foralltypes ofmonitoring,evenifitisaminimallyinvasivetechnique.It isimportanttomonitorthehemodynamicchangesovershort periods of time, and interventions should be made when necessary.Acontinuousmeasurementof allhemodynamic variablesispreferablebecauseonedoesnotwanttowaste timetocorrectanyinstability orachieve agoal.Monitors forcontinuousmonitoringofcardiacoutputarepreferred, although there are no data to support the superiority of cardiacoutputcontinuousmeasurement overintermittent monitoring.Thesemeasurescouldbejustified,however,if sudden changescould bedetected early and intervention couldreadilybeprovided.14
Whatcomorbiditiesarereducedassociatedwiththeuse ofGDT?
GRADE:1B
Response: Perioperative GDT reduces the following complications after surgery: infections; wounds; gas-trointestinal bleeding and cardiocirculatory failure; and pulmonar,neurological,renalandhematological insufficien-cies(Table5).
Arguments: Surgical procedures in high-risk patients are associated with high incidence of postoperative complications. It was proved that GDT significantly reducesthenumberofsurgicalpatientswithpostoperative complications. Thirty-one studies with 5292 participants were enrolled in a Cochrane publication of 201261 to
describethe effectsofincreasedperioperativeblood flow usingfluid with or without inotropic or vasoactive drugs. The number of patients with complications has been reducedthroughtheintervention, witha RRof0.68 (95% CI 0.58---0.80). Hospital stay was reduced in the treat-mentgroup,onaveraged,by16.1days(95%CI0.43---1.89; p=0.002).Inaddition,threemorbidityrateswerereduced byincreasingtheoverallbloodflow:kidneyfailure,witha RRof 0.71 (95%CI 0.57---0.90); respiratoryfailure, witha RRof0.51(95%CI0.28---0.93);andwound infections,with aRRof0.65(95%CI0.51---0.84).Thesedataindicatethatin 100patientsexposedtotreatment,itcanbeexpectedthat 13/100avoid acomplication, 2/100preventrenal impair-ment,5/100preventrespiratoryfailure,and4/100prevent postoperativewoundinfection.
An updated literature search, recently published by Pearse,15identified38trialsthatincluded6595participants,
Isthereagoodcost-benefitintheuseofGDTcompared tostandardtreatmenttoreducemortalityandmorbidity inhigh-risksurgicalpatients?
GRADE:1C
Response: GDT implementation in high-risk surgical patientsundergoingmajorelectivesurgeryiseffectiveboth clinicallyandintermsofcostcomparedtostandard treat-ment.Theimplementationofadditionalcostsmaybeoffset bysavingsfromcostreductionduetothereduction compli-cationratesandhospitalstay.62
Arguments:SeveralstudieshaveshownthatGDT imple-mentationin high-risksurgicalpatientswaseffectiveboth clinicallyandintermsofcost.Fenwick63comparedmethods
to optimize oxygen delivery (using adrenaline or dopex-amine)toreducethe risksassociatedwithmajorelective surgeryin high-riskpatientsandtocomparethecostsand cost-effectiveness of these approaches. The cost-benefit analysisrelatedthedifferenceincosttothedifferencein yearoflifegainedforafollow-upperiodoftwoyears.Ebm55
suggestedthatGDTinhigh-risksurgicalpatientsshouldbe thoroughlyexploredtocurbtheincreaseincostsassociated withmedicalcare.62,64
Discussion
Thefluidchallenge
A fluid challenge is one of the best tools that the anes-thetisthastoassessfluidresponsiveness.Forsuch,achange inpreload(fluidbolus)shouldbeinducedwhenmonitoring subsequentchangesin strokevolume,cardiacoutput,and dynamicindices,suchasPPV,SVVandPVI.65
Theuseofafluidbolusofferstwoadvantages:
(1) Awayofassessingtheresponseofapatienttofluidusing changesindynamicandstaticvolumeindices,flowand oxygenation;and
(2) A change in the increase of intravascular volumeand oftenanecessaryincreaseintheflow(cardiacoutput).
Afluidbolusisaprovocativetestofthecirculation, sim-ilarto the use ofa step function engineering todefine a system.A ‘‘test’’usinga smallamountof fluid(bolus) to assessthevolumeresponsivenesscanreducetheriskofan excessivelyliberalfluidstrategyandthepossibleeffectsof fluidoverload.Thesetoolshelpdeterminetherequirements forfurtherfluidtherapy,preventingdeleteriouseffectsof fluidoverloadthroughtheadministrationofsmallvolumes. Noteworthy,thefluidchallengetechniqueisatestofthe cardiovascularsystem;itallowsclinicianstoassesswhether apatienthasapreloadreservesufficienttoincreasestroke volumewithmorefluid.Fluidtherapyshouldbeconsidered (Rahbari)66 after a positive response toa fluid challenge.
In contrast to a single fluid challenge, fluid may also be administeredinacontrolledmannerbasedonanalgorithm, repeatingthefluidchallengeaslong asthereisapositive response.This controlledapproach is called ‘‘maximizing strokevolume’’andisthecornerstoneofmostgoal-directed therapyprotocols(Noblett).67Thus,theonlyreasonto
per-formafluidchallengeis toincreasethestroke volumeof apatient;if thisincrease does notoccur; itis likelythat
an additionaladministration of fluid is harmful. The only excessfluidthatcanbeadministeredinafluidchallengeis theamountusedtowhichthepatientdoesnotrespond.
Afluidchallengeshouldcomprisefourseparateorders: typeoffluidtobeadministered;volumeoffluidtobe admin-istered;infusionrate;andstoppingrulesifadverseeffects areseenbeforethefullamountofthebolusisadministered. Forrapidinfusionsofverysmallfluidbolus(e.g.,250mLof crystalloidfor1---2min),stoppingrulesareprobablynot nec-essary.However,iflargeramountsoffluidorlongerinfusion timesareused,itisimportanttohaveclearstoppingrules topreventrightheartfailureorpulmonaryedema.
Althoughthereisnoconsensusontypeorexactdosageof fluidadministration,bolusesaredeliveredfasteratarapid rate(5---10min)withaquickevaluationofthephysiological response.Themagnitudeofthisresponsehelpsdetermine the fluid challenge effectiveness, aswell as the require-mentsfor additionalfluidtherapies. Considering allthese aspects,thisapproachavoidsthedeleteriousconsequences offluidoverload.Thepeakandmaintenanceofthedynamic andstaticvariablesimprovementafterafluidbolusdepend bothonphysiological stateandfluidcomposition. Further-more, the response maintenance after the bolus may be reducedinthepresenceofcontinuedbleeding.
We recommend bolus therapy rather than continuous infusionwhentheaimistoimprovethepressure,perfusion, and oxygendelivery. Thereshould bea standard for fluid bolusinrelationtothecompositionandfluidvolume, infu-sion rate, and post-bolus evaluation time. Variables used to evaluatethe fluid bolus efficacy should include appro-priate changes incardiac outputor stroke volume and,if appropriate,dynamicindicesoffluidresponsiveness.
Limitationsofdynamicindices
Fluid responsiveness measurements cannot be usedin all patientsand,inmanyitcannotbeusedatalltimes.Dynamic indices have a high predictive value in determining the responsivenesstofluid;however, specificcriteriamust be met to use these indices to assess fluid responsiveness. Intraoperativemovements,electrosurgicalequipment,and physiological artifacts(noise)caninterferewiththe accu-rate interpretation of the dynamic indexes. Four primary limitationsmayexistintheuseofdynamicindexes.
interdependence, it will be observed a paradoxical posi-tive value ofPVI, PPV, SVV or PWV,which willbe further increasedupon fluidresuscitation. This increase isdue to positive pressure inspiration decreasing the end-diastolic volumeintheright ventricle,allowingincreasedleft ven-tricularfillingandthus agreaterstroke volume.However, thelargestpulsepressureandstroke volumeoccur during inspiration,whileinpatientsresponsivetovolume,greater pulsepressureandstrokevolumewilloccurduring exhala-tion.
As few clinicians control changes in flow and pressure onthe breathing phase,and it is known that the patient haspulmonaryhypertensionandcorpulmonale,itisbetter nottousedynamicindicesalonetoassessresponsivenessto volume.Recentstudieshavedemonstratedthatthe intraop-erativeuseofPPV/SVVwasinconclusiveinidentifying25%of patientsrequiringfluidtoundergogeneralanesthesia.68The
useoftheseindicesinICUpatientstoassessfluid responsive-nessisevenmoreproblematic,withonly2%ofICUpatients meetingthecriteria.69Therefore,whendynamicindicesare
usedtoguidefluidtherapy,somemeasuresofincreased per-fusioneffectivenessshouldbeconsidered.Insuchcases,and withthe limitationsofalldynamicindiceslisted above,it is indicated to perform a fluidchallenge or a passive leg elevationtesttoidentifythefluidresponsiveness.
Specifically, whenanyof theabovelimitations prevent the use of these parameters, one can consider perform-ingapassivelegelevationmaneuver(LEM).70 Incontrastto
mechanicalbreathing,whichusuallyreducesCO,LEMcause anendogenousfluidchallenge,which willincrease theCO in ‘‘responsive’’ patients. LEM maneuver have a sensitiv-ityof 89.4% andaspecificity of 91.4%for predicting fluid responsivenessand is bestcombinedwithminimally inva-sive cardiac output monitors that can control changes in strokevolumeandcardiacoutput,dynamicallyinrealtime, regardlessofventilationmode.71,72LEMexecution,however,
requiresamajorchangeof position,whichoften makesit impracticableforintraoperativeuse.
However,therearecasesintheoperatingroom(OR)in whichposturalchangescaninducehemodynamicresponse thatmayserve asadiagnosticmaneuver for fluid respon-siveness.
Implications
Werecommendthathemodynamicparametersareusedas anintegralpartofGDTprotocols.However,thelimitations ofeachdynamicindexmustbeconsidered.Thepresenceof fluidresponsivenessisnotanindicationforfluid administra-tion;thefinaldecision togivefluidmust besupportedby theclearneedforhemodynamicimprovement,presenceof fluidresponsiveness,andabsenceofassociatedrisks.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
TheauthorsandtheSociedadedeAnestesiologiadoEstado de São Paulo e a Sociedade Brasileira de Anestesiologia
wouldlikehighlightthefundamentalroleplayedbythe col-laboratorsAdrianaMarco Moussa,André SilvaMonteirode BarrosMaciel, Angela MigueldaSilva, Carla RobertaSilva deSouza, JuliaTomoeMimuraRodriguez,andPatriciados SantosBueno, in additionto thetechnical supportin the cost-effectivenessstudy ofAxiabio Consultoria Econômica Ltda.
References
1.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for predic-tionofcardiac riskofmajornoncardiacsurgery.Circulation. 1999;100:1043---9.
2.MehtaRL,KellumJA,ShahSV,etal.AcuteKidneyInjury Net-work: reportof an initiative to improve outcomes in acute kidneyinjury.CritCare.2007;11:R31.
3.CopelandGP,JonesD,WaltersM.POSSUM:ascoringsystemfor surgicalaudit.BrJSurg.1991;78:355---60.
4.McGinley A, Pearse RM. A national early warning score for acutelyillpatients.BMJ.2012;345:e5310.
5.VonlanthenR, Clavien P.What factors affectmortality after surgery?Lancet.2012;380:1034---6.
6.PearseRM,HarrisonDA,JamesP,etal.Identificationand char-acterizationofthehigh-risksurgicalpopulationintheUnited Kingdom.CritCare.2006;10:R81.
7.PearseRM,MorenoRP,BauerP,etal.Mortalityaftersurgeryin Europe:a7daycohortstudy.Lancet.2012;380:1059---65. 8.Lienhart A, Auroy Y, Péquignot F, et al. Survey of
anesthesia-related mortality in France. Anaesthesiology. 2006;105:1087---97.
9.Kirov MY,Kuzkov VV,Molnar Z.Perioperative haemodynamic therapy.CurrOpinCritCare.2010;16:384---92.
10.KehletH,WilmoreDW.Multimodalstrategiestoimprove surgi-caloutcome.AmJSurg.2002;183:630---41.
11.FossNB,KehletH.Mortalityanalysisinhipfracturepatients: implicationsfordesignoffutureoutcometrials.BrJAnaesth. 2005;94:24---9.
12.BenesJ,ChytraI,AltmannP,et al.Intraoperativefluid opti-mization using stroke volume variation in high risk surgical patients:resultsof prospectiverandomizedstudy.CritCare. 2010;14:R118.
13.Kehlet H, Bundgaard-Nielsen M. Goal-directed perioperative fluid management: why, when, and how? Anesthesiology. 2009;110:453---5.
14.VincentJL, RhodesA, PerelA, etal.Clinicalreview:update onhemodynamic monitoring--- a consensusof16. CritCare. 2011;15:229.
15.Pearse RM, Harrison DA, MacDonald N, et al. Effect of a perioperative, cardiac output-guided hemodynamic ther-apy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA.2014;311:2181---90.
16.CecconiM,CorredorC,ArulkumaranN,etal.Clinicalreview: goal-directedtherapy-whatistheevidenceinsurgicalpatients? Theeffectondifferentriskgroups.CritCare.2013;17:209. 17.FindlayGP,GoodwinAPL,ProtopapaA,etal.Knowingtherisk.
Areviewoftheperioperativecareofsurgicalpatients.London: NCEPOD;2011.
18.MichardF,BoussatS,ChemlaD,etal.Relationbetween respira-torychangesinarterialpulsepressureandfluidresponsiveness insepticpatientswithacutecirculatoryfailure.AmJRespir CritCareMed.2000;162:134---8.
and high-risk surgical patients. Anesth Analg. 2011;112: 1392---402.
20.CorcoranT,RhodesJE,ClarkeS,etal.Perioperativefluid man-agementstrategiesinmajorsurgery:astratifiedmeta-analysis. AnesthAnalg.2012;114:640---51.
21.BrandstrupB,SvendsenPE,RasmussenM,etal.Whichgoalfor fluidtherapyduringcolorectalsurgeryisfollowedbythebest outcome:near-maximalstrokevolumeorzerofluidobalance? BrJAnaesth.2012;109:191---9.
22.NisanevichV,Felsenstein I,AlmogyG, etal.Effect of intra-operativefluidmanagementonoutcomeafterintraabdominal surgery.Anaesthesiology.2005;103:25---32.
23.SrinivasaS,LemanuDP,SinghPP,etal.Systematicreviewand meta-analysisofoesophagealDoppler-guidedfluidmanagement incolorectalsurgery.BrJSurg.2013;100:1701---8.
24.National Heart, Lung, and BloodInstitute Acute Respiratory DistressSyndrome(ARDS)ClinicalTrialsNetwork,Wiedemann HP,Wheeler AP, etal. Comparison oftwo fluid-management strategies in acute lung injury. N Engl J Med. 2006;354: 2564---75.
25.WiedemannHP.Aperspectiveonthefluidsandcatheters treat-menttrial(FACTT).Fluid restrictionissuperiorinacutelung injuryandARDS.ClevelandClinJMed.2008;75:42---8. 26.PerelA,HabicherM,SanderM.Bench-to-bedsidereview:
func-tionalhemodynamicsduringsurgery---shoulditbeusedforall high-riskcases?CritCare.2013;17:203.
27.PerelA. Thevalueoffunctionalhemodynamicparametersin hemodynamicmonitoringofventilatedpatients.Anaesthesist. 2003;52:1003---4.
28.Boyd O, Jackson N.How is risk defined inhigh-risk surgical patientmanagement?CritCare.2005;9:390---6.
29.Giglio MT, Marucci M, Testini M, et al. Goal-directed haemodynamic therapy and gastrointestinal complicationsin majorsurgery:ameta-analysisofrandomizedcontrolledtrials. BrJAnaesth.2009;103:637---46.
30.AbbottT, AcklandGL.Therelationshipsbetweenanesthesia, hemodynamicsandoutcomes.In:CannessonM,PearseR, edit-ors.Perioperativehemodynamicmonitoringandgoaldirected therapy:fromtheorytopractice.Cambridge:Cambridge Uni-versityPress;2014[chapter26].
31.RhodesA, CecconiM,HamiltonM,etal. Goal-directed ther-apy inhigh-risksurgical patients: a 15-yearfollow-up study. IntensiveCareMed.2010;36:1327---32.
32.Guyatt GH, Oxman AD, VistGE, et al. GRADE: an emerging consensuson ratingquality ofevidenceand strengthof rec-ommendations.BMJ.2008;336:924---6.
33.GuyattGH,OxmanAD,KunzR,etal.Goingfromevidenceto recommendations.BMJ.2008;336:1049---51.
34.Schünemann HJ,Jaeschke R, CookDJ, et al.Anofficial ATS statement:gradingthequalityofevidenceandstrengthof rec-ommendationsinATSguidelinesandrecommendations.AmJ RespirCritCareMed.2006;174:605---14.
35.MillerTE,RocheAM,MythenM.Fluidmanagementand goal-directed therapy as an adjunct to enhanced recovery after surgery(ERAS).CanJAnaesth.2015;62:158---68.
36.Moonesinghe SR,Mythen MG,Das P,et al. Riskstratification toolsforpredicting morbidityand mortalityinadultpatients undergoingmajorsurgery:qualitativesystematicreview. Anes-thesiology.2013;119:959---81.
37.PearseR,DawsonD,FawcettJ,etal.Earlygoal-directed ther-apyaftermajorsurgeryreducescomplicationsanddurationof hospitalstay.Arandomized,controlledtrial[ISRCTN38797445]. CritCare.2005;9:R687---93.
38.BisgaardJ,GilsaaT,RønholmE,etal.Optimizingstrokevolume andoxygendeliveryinabdominalaorticsurgery:arandomized controlledtrial.ActaAnaesthesiolScand.2013;57:178---88. 39.AcklandGL,IqbalS,ParedesLG,etal.Individualizedoxygen
deliverytargetedhaemodynamictherapy inhigh-risksurgical
patients:amulticentre,randomized,double-blind,controlled, mechanistictrial.LancetRespirMed.2015;3:33---41.
40.Cecconi M, Fasano N, Langiano N, et al. Goal-directed haemodynamictherapy duringelectivetotalhiparthroplasty underregionalanaesthesia.CritCare.2011;15:R132.
41.ShoemakerWC,AppelPL,KramHB,etal.Prospectivetrialof supranormalvaluesofsurvivorsastherapeuticgoalsinhigh-risk surgicalpatients.Chest.1988;94:1176---86.
42.SinclairS,JamesS,SingerM.Intraoperativeintravascular vol-umeoptimizationand lengthofhospital stayafterrepair of proximalfemoralfracture: randomizedcontrolledtrial.BMJ. 1997;315:909---12.
43.LoboSM,SalgadoPF,CastilloVG,etal.Effectsofmaximizing oxygendeliveryonmorbidityandmortalityinhigh-risksurgical patients.CritCareMed.2000;28:3396---404.
44.LopesMR,OliveiraMA,Pereira VO,etal.Goal-directedfluid managementbasedonpulsepressurevariationmonitoring dur-inghigh-risksurgery:apilotrandomizedcontrolledtrial.Crit Care.2007;11:R100.
45.BarthaE,ArfwedsonC,ImnellA,etal.Randomizedcontrolled trialofgoal-directedhaemodynamictreatmentinpatientswith proximalfemoralfracture.BrJAnaesth.2013;110:545---53. 46.BisgaardJ,GilsaaT,RønholmE,etal.Haemodynamic
optimiza-tioninlowerlimbarterialsurgery:roomforimprovement?Acta AnaesthesiolScand.2013;57:189---98.
47.DonatiA,LoggiS,PreiserJC,etal.Goal-directedintraoperative therapyreducesmorbidityandlengthofhospitalstayin high-risksurgicalpatients.Chest.2007;132:1817---24.
48.Scheeren TW, Wiesenack C,Gerlach H, et al. Goal-directed intraoperativefluidtherapyguidedbystroke volumeand its variationinhigh-risksurgicalpatients:aprospective random-izedmulticentrestudy.JClinMonitComput.2013;27:225---33. 49.GanTJ,SoppittA,MaroofM,etal.Goal-directedintraoperative
fluidadministrationreduceslengthofhospitalstayaftermajor surgery.Anaesthesiology.2002;97:820---6.
50.Mayer J, Boldt J, Mengistu AM, et al. Goal- directed intra-operative therapy based on autocalibratedarterial pressure waveform analysis reduces hospital stay in high-risk sur-gical patients: a randomized, controlled trial. Crit Care. 2010;14:R18.
51.LoboSM,LoboFR,PolachiniCA,etal.Prospective,randomized trialcomparingfluidsanddobutamineoptimizationofoxygen deliveryin high-risksurgicalpatients[ISRCTN42445141]. Crit Care.2006;10:R72.
52.ChallandC,StruthersR,SneydJR,etal.Randomizedcontrolled trialof intraoperative goal-directedfluid therapy in aerobi-callyfitandunfitpatientshavingmajorcolorectalsurgery.BrJ Anaesth.2012;108:53---62.
53.Agency for Healthcare Research and Quality. Esophageal Doppler ultrasound-based cardiac output monitoring for real-time therapeutic management of hospitalized patients; 2007 [accessed 21 January 2016], http://www.cms.gov/ determinationprocess/downloads/id45TA.pdf
54.Health and Clinical Excellence. CardioQ-ODM oesophageal Doppler Monitor; 2011 [accessed 21 January 2016], http://www.nice.org.uk/nicemedia/live/13312/52624/52624. pdf
55.EbmC,CecconiM,SuttonL,etal.Acost-effectiveness analy-sisofpostoperativegoal-directedtherapyforhigh-risksurgical patients.CritCareMed.2014;42:1194---203.
56.Cuthbertson BH, Campbell MK,Stott SA, et al. A pragmatic multi-centrerandomizedcontrolledtrialoffluidloadingin high-risksurgicalpatientsundergoingmajorelectivesurgery---the FOCCUSstudy.CritCare.2011;15:R296.
58.PerelA,PizovR,CotevS.Respiratoryvariationsinthearterial pressureduring mechanicalventilationreflectvolume status andfluidresponsiveness.IntensiveCareMed.2014;40:798---807. 59.Cannesson M, Ramsingh D, Rinehart J, et al. Periopera-tive goal-directed therapy and postoperative outcomes in patientsundergoinghigh-riskabdominalsurgery:a historical-prospective, comparative effectiveness study. Crit Care. 2015;19:261.
60.NavarroLH, BloomstoneJA,AulerJOJr,et al.Perioperative fluidtherapy:astatementfromtheinternationalfluid optimiza-tiongroup.PerioperMed(Lond).2015;4:3.
61.Grocott MP, Dushianthan A, Hamilton MA, et al. Periopera-tive increase in global blood flow to explicit defined goals andoutcomesfollowingsurgery.CochraneDatabaseSystRev. 2012:CD004082.
62.BarthaE,DavidsonT,HommelA,etal.Cost-effectiveness anal-ysis ofgoal-directed hemodynamic treatment of elderly hip fracturepatients:beforeclinicalresearchstarts. Anesthesiol-ogy.2012;117:519---30.
63.FenwickE, Wilson J, Sculpher M, et al. Pre-operative opti-mization employing dopexamina or adrenaline for patients undergoingmajorelectivesurgery:acost-effectiveness anal-ysis.IntensiveCareMed.2002;28:599---608.
64.Manecke GR, Asemota A, Michard F. Tackling the economic burdenofpostsurgicalcomplications:wouldperioperative goal-directedfluidtherapyhelp?CritCare.2014;18:566---74.
65.CherpanathTG,GeertsBF,LagrandWK,etal.Basicconceptsof fluidresponsiveness.NethHeartJ.2013;21:530---6.
66.RahbariNN,ZimmermannJB,SchmidtT,etal.Meta-analysisof standard,restrictiveandsupplementalfluidadministrationin colorectalsurgery.BrJSurg.2009;96:331---41.
67.NoblettSE,SnowdenCP,ShentonBK,etal.Randomizedclinical trialassessingtheeffectofDoppler-optimizedfluid manage-mentonoutcomeafterelectivecolorectalresection.BrJSurg. 2006;93:1069---76.
68.CannessonM,LeManachY,HoferCK,etal.Assessingthe diag-nosticaccuracyofpulsepressurevariationsforthepredictionof fluidresponsiveness:a‘‘grayzone’’approach.Anesthesiology. 2011;115:231---41.
69.Holte K, Sharrock NE, Kehiet H. Pathophysiology and clini-cal implicationsof perioperative fluid excess. Br JAnaesth. 2002;89:622---32.
70.GuerinL, MonnetX, Teboul JL. Monitoringvolumeand fluid responsiveness:from statictodynamicindicators. BestPract ResClinAnaesthesiol.2013;27:177---85.
71.MarikPE,LemsonJ.Fluidresponsiveness:anevolutionofour understanding.BrJAnaesth.2014;112:620---2.