www.jped.com.br
ORIGINAL
ARTICLE
Varicella
zoster
virus
related
deaths
and
hospitalizations
before
the
introduction
of
universal
vaccination
with
the
tetraviral
vaccine
夽
Alessandra
de
Martino
Mota
a,
Filipe
Anibal
Carvalho-Costa
a,b,∗aLaboratóriodeEpidemiologiaeSistemáticaMolecular,InstitutoOswaldoCruz(IOC),Fundac¸ãoOswaldoCruz(Fiocruz),
RiodeJaneiro,RJ,Brazil
bEscritórioRegionalFiocruzPiauí,Fundac¸ãoOswaldoCruz(Fiocruz),Teresina,PI,Brazil
Received16June2015;accepted21October2015 Availableonline8March2016
KEYWORDS
Varicella-zostervirus; Varicella;
Tetraviralvaccine; Deaths;
Hospitalizations
Abstract
Objective: Tocharacterizevaricellazostervirus-relateddeathsandhospitalizationsinBrazil before universal vaccinationwith the tetravalent (measles, mumps, rubella,and varicella) vaccine,attemptingtocollectbaselinedataonvaricellamorbidityandmortalityinorderto evaluatetheimpactofthevaricellavaccinationprogram.
Methods: Varicella-associatedmortalitydatawereevaluatedbetween1996and2011and vari-cellazostervirus-associatedhospitalizationsbetween1998and2013.Dataweregatheredfrom theInformaticsDepartmentoftheUnifiedHealthSystem,consideringtheInternational Classi-ficationofDiseases,10thRevision,codeB01.Allagegroupswereassessed.Varicella-specific mortalityrateswerecalculatedandseasonalityofvaricella-zostervirus-associated hospitaliza-tionswasdescribed.
Results: Therewere2334varicelladeathsbetween1996and2011,19.3%ininfantsagedless than1yearand36%inchildrenfrom1to4years.Ininfantsunder1year,varicellamortality ratesreached3.2/100,000/year.In children aged1---4 years,varicella mortalityratesreach 1.64/100,000/year.AverageannualmortalityratesforvaricellainBrazilare0.88/100,000in infantsunder1yearand0.40/100,000inchildrenaged1---4years.Thetotalnumberof hospital-izationsassociatedwithvaricellazosterviruswas62,246from2008to2013.Varicella-associated hospitalizationshaveaseasonaldistributioninchildren,peakinginNovember.Intheelderly, monthlyaveragesofherpeszoster-associatedhospitalizationspresentnosignificantseasonal variation.
夽
Pleasecitethisarticleas:Martino MotaA,Carvalho-Costa FA. Varicellazostervirusrelateddeathsandhospitalizationsbeforethe introductionofuniversalvaccinationwiththetetraviralvaccine.JPediatr(RioJ).2016;92:361---6.
∗Correspondingauthor.
E-mail:guaratiba@ioc.fiocruz.br(F.A.Carvalho-Costa).
http://dx.doi.org/10.1016/j.jped.2015.10.003
Conclusions: Varicella isassociated, inthe pre-vaccineperiod, tosignificant morbidityand mortalityin Brazil.The universal vaccination program isexpected todecrease the disease burdenfromvaricella.
©2016SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/ 4.0/).
PALAVRAS-CHAVE
Vírusvaricela-zoster; Varicela;
Vacinatetravalente; Óbitos;
Internac¸ões
Óbitoseinternac¸õesrelacionadosaovírusvaricela-zosterantesdaintroduc¸ãoda
vacinac¸ãouniversalcomavacinatetravalente
Resumo
Objetivo: Caracterizarosóbitoseinternac¸õesrelacionadosao vírusvaricela-zosternoBrasil antesdavacinac¸ãouniversalcomavacinatetravalente(sarampo,caxumba,rubéolaevaricela), tentandocoletardadosdereferênciasobreamorbidezemortalidadeporvaricela,paraavaliar oimpactodoprogramadevacinac¸ãocontraavaricela.
Métodos: Osdadosdemortalidadeassociadaàvaricelaforamavaliadosentre1996e2011eas internac¸õesassociadasaovírusvaricela-zoster,entre1998e2013.Osdadosforamcoletados doDepartamentodeInformáticadoSistemaUnificadodeSaúde,considerandoaClassificac¸ão Internacionalde Doenc¸as,10a Revisão, códigoB01.Todasasfaixas etáriasforamavaliadas.
Foramcalculadasastaxasdemortalidadeespecíficasporvaricelaefoidescritaasazonalidade dasinternac¸õesassociadasaovírusvaricela-zoster.
Resultados: Houve2.334óbitosporvaricelaentre1996e2011,19,3%emneonatoscommenos de1ano e36%em crianc¸asde1a4anos.Em neonatos commenosde1ano, astaxasde mortalidadepor varicelaatingiram 3,2/100.000/ano.Emcrianc¸asde 1---4anosdeidade,as taxasdemortalidadeporvaricela atingem1,64/100.000/ano.Astaxasde mortalidade anu-ais médiasporvaricela noBrasil sãode0,88/100.000emneonatos commenos de1ano de idadee0,40/100.000emcrianc¸asde1a4anosdeidade.Onúmerototaldeinternac¸ões asso-ciadas ao vírusvaricela-zosterfoide 62.246de2008a2013. Asinternac¸õesrelacionadasà varicelaapresentaramdistribuic¸ãosazonalemcrianc¸as,compicoemnovembro.Emidosos,as médiasmensaisdeinternac¸õesassociadasaoherpeszosternãoapresentamvariac¸ãosazonal significativa.
Conclusões: Avaricelaestáassociadaamorbidezemortalidadesignificativasnoperíodo pré-vacinac¸ãonoBrasil.Oprogramadevacinac¸ãouniversaldevediminuir acargadedoenc¸ada varicela.
©2016SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Este ´eumartigo OpenAccesssobumalicenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4. 0/).
Introduction
Varicella(chickenpox)isanacute,exanthematicand conta-giousinfectiousdiseasethatoccursprimarilyinchildhood.1
Itiscausedbyvaricellazostervirus(VZV),analphaherpes virusbelongingtotheherpesviridaefamily.2---4After
resolu-tionofchickenpox,VZVremainslatentindorsalrootspinal gangliaandreactivationcanariseatanystageoflife,more frequentlyatanolderage,causingherpeszoster.2,3,5---7
Although it is generally considered a mild childhood disease, varicella can be severe in children, adults, and immunocompromised individuals8 due torisk of viral
dis-seminationto internal organs, such as lungs,liver, brain, heart,andkidneys.Themostfrequentcomplicationsof vari-cella are secondary bacterial infections caused by Group A -hemolyticus Streptococcus or Staphylococcus aureus, usuallyaffectingthe skinandsoft tissues.Invasive bacte-rialinfectionssuchaspneumonia,arthritis,osteomyelitis, sepsis, and necrotizing fasciitis may be fatal.1
Neurologi-calcomplications such ascerebellar ataxia, encephalitis, meningitis,andvasculitiscanalsooccur.9
The live attenuated vaccine against varicella was for-mulated in Japan in 1974.10 Varicella vaccines, available
worldwide,possessthesingleVZVorarecombinedwiththe measles,mumps,andrubellaviruses,formingatetravalent vaccine(MMRV).6AccordingtoOzaki,thepreventiveeffect
of varicellavaccine was estimated at 75%. In the United Statesitranged between79%and88%withthefirstdose, forallformsofthedisease.6
In2013, a productdevelopment partnershipunder the Brazilian Ministry of Health involving the pharmaceutical industry enabled the production of MMRV at the National Institute of Immunobiological Technology(Biomanguinhos) oftheOswaldoCruzFoundation(Fiocruz,RJ,Brazil).Thus, universalvaccinationagainstvaricellabeganinSeptember 2013,inBrazil,throughtheNationalImmunizationProgram (NIP).AsingledoseofMMRV isadministeredat theageof 15months;forinfantsreceivingtheMMR(measles,mumps, andrubella),atage12months.
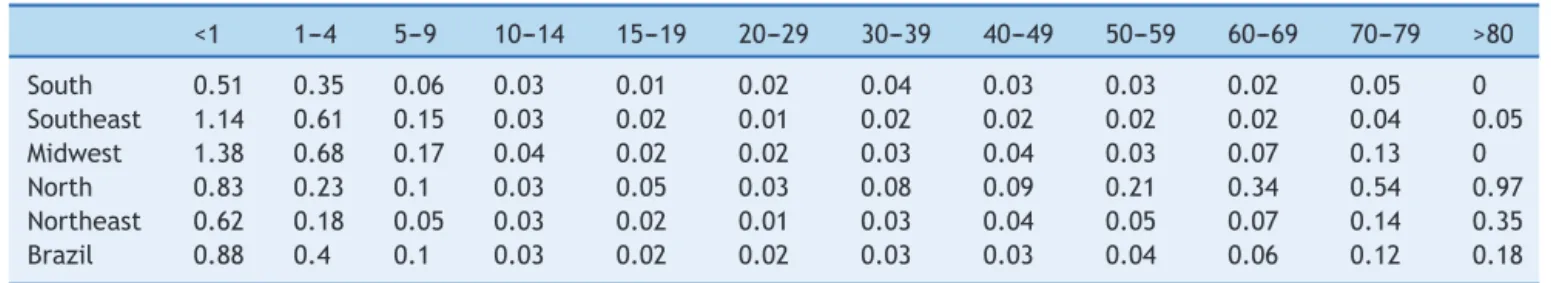
Table1 Averageyearmortalityratesforvaricellaper100,000byagegroupinBrazilianregions,1996---2011.
<1 1---4 5---9 10---14 15---19 20---29 30---39 40---49 50---59 60---69 70---79 >80
South 0.51 0.35 0.06 0.03 0.01 0.02 0.04 0.03 0.03 0.02 0.05 0
Southeast 1.14 0.61 0.15 0.03 0.02 0.01 0.02 0.02 0.02 0.02 0.04 0.05
Midwest 1.38 0.68 0.17 0.04 0.02 0.02 0.03 0.04 0.03 0.07 0.13 0
North 0.83 0.23 0.1 0.03 0.05 0.03 0.08 0.09 0.21 0.34 0.54 0.97
Northeast 0.62 0.18 0.05 0.03 0.02 0.01 0.03 0.04 0.05 0.07 0.14 0.35
Brazil 0.88 0.4 0.1 0.03 0.02 0.02 0.03 0.03 0.04 0.06 0.12 0.18
aims to describe the frequency and seasonal distribution ofvaricella-associateddeaths,aswellasthefrequencyof VZV-relatedhospitalizationsinthepre-vaccinationperiodin Brazil.
Materials
and
methods
Varicella-associated mortality data and VZV-associated hospitalizationsweregatheredfromtheInformatics Depart-ment of the Unified Health System (DataSUS), through the website http://www2.datasus.gov.br/DATASUS/index. php?area=02.OntheHealthInformationpage,theMortality sectionof VitalStatisticswasaccessed in ordertoreview varicella-relateddeaths. TheHospitalMorbiditysectionin Epidemiological Information and Morbidity was accessed in order to assess VZV-related hospitalizations. Varicella-relateddeathswereverifiedaccordingtotheInternational ClassificationofDiseases,10thRevision(ICD-10)codeB01.
VZV-associated hospitalizations wereverifiedaccording to an uncoded morbidity list (ICD-10). Varicella deaths comprised the period1996---2011, and hospitalizations for varicellaand herpeszoster from2008 to2013 were stud-ied.Whendatacollectionwasperformed,thelatestperiod available for death records was prior 2011, and for hos-pitalizations, before 2013. Moreover, in the period prior to 1996, the age-related data were uneven. The authors consideredbothvaricellaandherpeszosterfor hospitaliza-tionsbecauseDataSUSdoesnotdiscriminatehospitalization records between varicella and herpes zoster. Varicella-relateddeathswereevaluatedbecausethediseaseismore frequentinchildren.
Varicella-specific mortality in age groups was calcu-lated as the number of varicella deaths in infants aged less than 1 year×100,000/population less than 1 year,
andin childrenaged1---4years×100,000/populationaged
1---4 years in five Brazilian macro-regions (North, North-east,South,Southeast,andMidwest).Populationsizeused inthedenominatorswasobtainedfromtheBrazilian Insti-tute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística [IBGE]) based on the 1991, 2000, and 2010censuses and respectiveannual intercensal pro-jections.SeasonalityofVZV-associatedhospitalizationswas evaluated through the averages and standard deviations of the monthly number of VZV-associatedhospitalizations from 2008 to 2013 in the age groups. It was assumed that VZV-associated hospitalizations among infants aged under1 year and 1---4 yearsarepredominantly relatedto varicella, while hospitalizations among adults older than
65 years old are mainly caused by herpes zoster. The studywas submittedtothe Ethics Committeeof Oswaldo CruzInstitute and approvedwith referencenumber CAAE 27704214.3.0000.5248.
Results
Therewere2334varicella-associateddeathsbetween1996 and 2011 in Brazil. From these, 19.3% (n=450) were in infantsagedlessthanoneyear,36%(n=840)werein chil-drenaged1---4 years,11.7%(n=273) inchildren aged5---9 years,and33%(n=771)inpatientsmorethan9yearsold.
Table1 shows theaverage mortality rates for varicellain agegroupsindistinctBrazilianregions,from1996to2011. Ininfantsunder1yearofage,varicellamortalityratesvary indistinctBrazilianregions,from0to3.2/100,000/year.In childrenaged1---4years,varicellamortalityratesvaryfrom 0to1.64/100,000/year.Averageannualmortalityratesfor varicellainBrazilfrom1996to2011were0.88/100,000in infantsunder1yearand0.40/100,000inchildrenaged1---4 years.
The number of VZV-associated hospitalizations by age group in the periodbefore MMRV vaccine introduction in Brazilfrom2008to2013isrepresentedinFig.1.The major-ityofhospitalizationsemergedinchildrenunder9yearsof age,withpeaksintheagegroup1---4yearsandintheelderly after80yearsofage.Thepresentstudydemonstratesthat thefrequency ofhospitalizations for herpes zosterranges from4378,5084,and5151casespermonthinBrazilinthe age groups 60---69, 70---79, and over 80 yearsold, respec-tively.Thetotalnumberofhospitalizationsassociatedwith
0
1 to 4 0 to 1 5 to 9
10 to 1415 to 1920 to 2425 to 2930 to 3435 to 3940 to 4445 to 4950 to 5455 to 5960 to 6465 to 6970 to 7475 to 79 80 +
2000 4000 6000 8000 10 000 12 000 14 000 16 000 18 000
Number of hospitalizations
Age category (years)
250
A
B
C
D
Months
Number of hospitalizations
Number of hospitalizations
Number of hospitalizations
Number of hospitalizations
200
150
100
100 200 300 400 500 600 700
0
0
0 10 20 30 40 50 60 70 10 20 30 40 50 60 50
0
Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec
Months
Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec
Months
Mean 1SD 2SD
Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec
Months
Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec
Figure2 Monthlyaverages ofhospitalizations for varicella indifferentagecategories,showingaseasonaldistributionin children(A,B)andnovariationinadults(C,D).(A)Infantsaged under1year.(B)Childrenaged1---4years.(C)Adultsaged65---69 years.(D)Adultsaged75---79years.
VZV was 62,052, and varicella-associated hospitalizations hadaseasonaldistributioninchildren,peakinginNovember. Inadultsolderthan65years,monthlyaveragesof herpes-zoster-associated hospitalizations presented no variation. Averages and standard deviations of monthly numbers of hospitalizationsfrom2008to2013aredepictedinFig.2A---D.
Discussion
Thisstudydemonstratedsignificantmorbidityandmortality fromvaricellainBrazilpriortotheintroductionofMMRVinto theNIP.Datasuggestthatvaricellashouldnotbeconsidered asabenigndisease,asitpresentsfrequentcomplications. Althoughthemajorityofdeathswereinchildrenaged1---4 years,themortalityrateishigherininfantsagedlessthan 1 year. Data suggest that the risk of a child dying from varicellaistwiceashighforinfantsunder1yearthan chil-dren aged 1---4 years. However,the MMRV vaccine in the BrazilianNIPisgiventochildrenaged15months.Thus, vari-cella wouldnot beprevented in youngerinfants. In some countriespracticinguniversalvaricellavaccination,the vac-cineisadministeredearlier,at12monthsofage,asinthe United States, Canada, Japan, China, and Uruguay.6,11---14
Importantly,thecohortofimmunizedchildrenwillincrease progressively over the next years, putatively leading to reducedVZVcirculationamongimmunizedchildren,which will benefit children less than 1 year old by herd immu-nity,asdescribedbyStrengetal.15 However,thepotential
reactivationofVZVinadultsrepresentsacontinuous repos-itoryofwildstrains.Inthiscase,childrenunder15months oldwillbesusceptibletoacquiringtheinfection.
Althoughtherewasa significantreduction in varicella-associatedhospitalizations incountriesthat haveadopted universal vaccination, like the United States, outbreaks havestillbeenreported,evenamongpopulationswithhigh single-dose coverage. In the United States, the Advisory Committee on Immunization Practices passed the recom-mendation for routine two-dose varicella vaccination in 2006,whichwas10yearsaftertheone-dosevaricella vac-cination program started in the United States. Moreover, theseconddosewasrecommendednotonlyforoutbreaks, but alsotoincrease protection againstvaricella.16
Conse-quencesofvaccinefailurearepotentiallyseriousduetothe continuoustransmissionofwild VZVandtheaccumulation ofsusceptibleyoungadults.17Consequently,theneedfora
seconddoseofvaricellavaccineinsomecountrieshasbeen considered.18 Nevertheless,thehighmorbidityand
mortal-ity in children aged1---4 yearsin Brazil demonstrates the potential positiveimpacts ofa singleMMRV dose givenat theageof15months.
AccordingtoGoldmanandKing,19 therehasbeena
sig-nificant increase in the incidence of herpes zoster over the years following implementation of universal vaccina-tionagainst VZVin theUnited States,concomitantlywith the decline invaricella incidence.Therefore, it hasbeen arguedthatthepotentialincreaseintheincidenceof her-peszostercouldnullifythebenefitsofvaccination.19,20This
increasewouldberelatedtothelowcirculationofwildVZV inavaccinatedpopulation,whichrepresentsavaccination booster, increasing theimmune response toVZV and pre-ventingviral reactivationin the nervoussystem.19,21 Data
fromthepresentstudyprovideabaselineforcomparingthe post-vaccinationperiod.
Despite the existence of vaccination programs in sev-eral developed and developing countries, a question remains: whether or not to vaccinate children against varicella?
representingalmost onedeatheverytwodaysby the dis-ease,generatinganaverageof34hospitalizationsperday.In thepopulationofchildrenaged1---4years,anaverageofnine hospitalizationsperdaybythediseasehasbeenreported. Thisscenarioleadstheauthorstobelievethatvaricellain developingcountries---inconjunctionwithfactorssuchas poverty, malnutrition, and lack of access to health care --- frequentlyrequires hospitalization,possiblyresultingin death. In addition,the indirectcosts of the diseasemust beconsidered, suchasparental workleave and caregiver expense.Theestimatedaveragecostofworkdaylossfora motherwithachildunder15 yearsoldwithvaricellawas US$5.90,in2004.22
Inmustbeemphasizedthatduring14years,varicella vac-cinationwasavailableinprivatevaccinationclinicswidely usedbythepopulationofthemiddleandupperclassesin Brazil. However,therewascirculation of wild VZV in this period, which exposed the vaccinated population to the boostersthatprevented weaningof immunityafter vacci-nation.
Varicellahadalwaysbeenassociatedinthepre-vaccine period toa significant number of deaths and hospitaliza-tions. The introduction of MMRV into the Brazilian NIP generatedexpectationsofapositiveimpact,especiallyfor children.
Varicella-specificmortalityratesvaryindifferentyears. Theserates ininfantsunderoneyeararetwiceashighas those observed in children aged 1---4 years, although the absolutenumberisgreaterintherangeof1---4years.This indicates thata child under1 yearhas doublethe riskof dyingfromchickenpoxwhencomparedtochildrenaged1---4 years. Varicella-specific mortality rates are higher in the Brazilian Midwest and Southeast. VZV-related hospitaliza-tionshaveabimodaldistribution,peakinginchildrenunder 9yearsoldandpersonsolder than80.Thereisaseasonal patterninhospitalizationsfor varicellainchildren,witha highermonthlyaveragefromSeptembertoNovember;this is not observed in adults, which may be related to non-seasonalVZV-reactivationassociatedwithherpeszoster.
This survey has some limitations, because it is a ret-rospective study using secondary data. Furthermore, the diagnosis of varicella and herpes zoster are eminently clinical and varicella was not a reportable disease in all Brazilian states until September 2013. Therefore, it is possible that the disease is underdiagnosed, especiallyin country’sregions whereaccesstohealth careprogramsis stillprecarious.
Because varicella in Brazil is a disease that leads to almostonedeatheverytwodaysandbecauseithasaworse prognosisindevelopingcountries,theauthorsconcludethat universalvaricellavaccinationisfullyjustifiedinthe coun-try. An epidemiologic follow-up of breakthrough varicella andherpeszostercasesthatwillpossiblyoccurmoreoften in the coming yearsis essential, and it is vital todiscuss the inclusionof a second dose of varicella vaccine in the BrazilianNIP.
Funding
ThisstudywassupportedwithfundsfromFiocruz.
Conflicts
of
interest
Carvalho-CostaisapublichealthresearcheratOswaldoCruz Foundation (Fiocruz).The others authorsdeclare no con-flictsofinterest.
References
1.Bozzola E,Tozzi AE, BozzolaM, Krzysztofiak A, ValentiniD, Grandin A, et al. Neurological complications of varicella in childhood:caseseriesandasystematicreviewoftheliterature. Vaccine.2012;30:5785---90.
2.HeiningerU,SewardJF.Varicella.Lancet.2006;368:1365---76. 3.ArvinAM.Varicella-zostervirus.In:LongS,PickeringL,Prober
C,editors.Principlesandpracticeofpediatricinfectious dis-ease.Philadelphia:ElsevierHealthSciences;2012.p.1035---44. 4.FerreiraRA,PereiraAC.Varicela.In:CouraJR,editor.Dinâmica dasdoenc¸asinfecciosaseparasitárias,v.2,2ed.RiodeJaneiro: GuanabaraKoogan;2013.p.1951---4.
5.Pe˜na-ReyI,MartínezdeAragónMV,VillaverdeHuesoA,Terres ArellanoM,AlcaldeCaberoE,SuárezRodríguezB.Epidemiology ofvaricellainSpainpre-andpost-vaccinationperiods.RevEsp SaludPublica.2009;83:711---24.
6.Ozaki T. Varicella vaccination in Japan: necessity of imple-mentinga routine vaccinationprogram. JInfect Chemother. 2013;19:188---95.
7.BreuerJ,FiferH.Chickenpox.BMJClinEvid.2011;2011:0912. 8.PrebludSR.Age-specificrisksofvaricellacomplications.
Pedi-atrics.1981;68:14---7.
9.ScienceM,MacGregorD,RichardsonSE,MahantS,TranD, Bit-nunA.Centralnervoussystemcomplicationsofvaricella-zoster virus.JPediatr.2014;165:779---85.
10.TakahashiM,OtsukaT,OkunoY,AsanoY,YazakiT.Livevaccine usedtopreventthespreadofvaricellainchildreninhospital. Lancet.1974;2:1288---90.
11.QuianJ,RüttimannR,RomeroC,Dall’OrsoP,CerisolaA,Breuer T,etal.Impactofuniversalvaricellavaccinationon1-year-olds inUruguay:1997---2005.ArchDisChild.2008;93:845---50. 12.MarinM,BroderKR,TemteJL,SniderDE,SewardJF,Centers
forDiseaseControlandPrevention(CDC).Useofcombination measles, mumps, rubella, and varicella vaccine: recommen-dationsoftheAdvisoryCommitteeonImmunizationPractices (ACIP).MMWRRecommRep.2010;59:1---12.
13.LuL,WangC,SuoL,LiJ,LiuW,PangX,etal.Varicelladisease inBeijing intheeraofvoluntary vaccination,2007to 2010. PediatrInfectDisJ.2013;32:e314---8.
14.Russell ML,DoverDC,SimmondsKA, Svenson LW.Shinglesin Alberta:beforeandafterpubliclyfundedvaricellavaccination. Vaccine.2014;32:6319---24.
15.Streng A, Grote V, CarrD, Hagemann C,Liese JG. Varicella routine vaccination and the effects on varicella epidemiol-ogy---resultsfromtheBavarianVaricellaSurveillanceProject (BaVariPro),2006---2011.BMCInfectDis.2013;13:303. 16.Baxter R, Tran TN, Ray P, Lewis E, Fireman B, Black S,
etal.Impactofvaccinationontheepidemiologyofvaricella: 1995---2009.Pediatrics.2014;134:24---30.
17.GershonAA,KatzSL.Perspectiveonlive varicellavaccine.J InfectDis.2008;197:S242---5.
18.Prymula R, Bergsaker MR, Esposito S, Gothefors L, Man S, SnegovaN,etal.Protection againstvaricellawithtwodoses of combined measles-mumps-rubella-varicella vaccine versus one dose of monovalent varicella vaccine: a multicentre, observer-blind,randomised,controlledtrial.Lancet.2014;383: 1313---24.
cost-effectiveness,andvaccineefficacybasedprimarilyonthe AntelopeValleyVaricellaActiveSurveillanceProjectdata. Vac-cine.2013;31:1680---94.
20.ReynoldsMA,ChavesSS,HarpazR,LopezAS,SewardJF.The impactofthevaricellavaccinationprogramonherpeszoster epidemiology in the United States: a review. J Infect Dis. 2008;197:S224---7.
21.BrissonM,GayNJ,EdmundsWJ,AndrewsNJ.Exposureto vari-cellaboostsimmunitytoherpes-zoster:implicationsformass vaccinationagainstchickenpox.Vaccine.2002;20:2500---7. 22.Valentim J, Sartori AM, de Soárez PC, Amaku M, Azevedo