rev bras hematol hemoter. 2016;38(1):82–85
w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Case
Report
A
difficult
case
of
angioimmunoblastic
T-cell
lymphoma
to
diagnose
Elisabetta
Sachsida-Colombo
∗,
Livia
Caroline
Barbosa
Mariano,
Fernanda
Queiróz
Bastos,
Amanda
Bruder
Rassi,
Luís
Alberto
de
Pádua
Covas
Lage,
Ariel
Barreto,
Sheila
Siqueira,
Juliana
Pereira
UniversidadedeSãoPaulo(USP),SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received6May2015 Accepted10November2015 Availableonline14December2015
Introduction
AngioimmunoblasticT-celllymphoma(AITL)isamalignancy ofmatureT-cells.Itischaracterizedasapolymorphic
lym-phonodal lymphoid infiltrate accompanied by prominent
proliferation of endothelial venules and follicular den-dritic cells. AITL was first described in 1974 by Frizzera
et al. as an angioimmunoblastic lymphadenopathy with
dysproteinemia.1Ashorttimelater,thenamewaschangedto
immunoblasticlymphadenopathy,andthento lymphogranu-lomatosisXin1979.2
AITLcomprises15–20%ofallperipheralT-celllymphomas and 1–2% of all non-Hodgkin lymphomas(NHL). Most fre-quently, it occurs in aged patients, with equal prevalence between males and females. Typically, AITL displays an aggressivebehavior,whichmakesthediagnosisdifficultandit mustbedifferentiatedfromothermalignant lymphoprolifera-tivediseases,drugreactionsandviralinfections.Patientswith AITL frequentlyexhibitB-symptoms (e.g.,feverand weight loss)andageneralizedenlargementofthelymphnodes.Other
∗ Correspondingauthorat:CancerInstituteoftheStateofSãoPauloOctavioFriasdeOliveira(ICESP),UniversidadedeSãoPaulo(USP),
Av.Dr.Arnaldo,251CerqueiraCésar,01246-000SãoPaulo,SP,Brazil. E-mailaddress:sachsidacolombo@yahoo.com.br(E.Sachsida-Colombo).
common symptoms include hepatomegaly, splenomegaly,
polymorphicskinrashandpleuraleffusion.Advancedstage disease (AnnArborIII/IV)isobservedin80%ofcases.AITL isalsoassociatedwithautoimmunephenomena.Polyclonal
hypergammaglobulinemia occurs in approximately 50% of
AITLcases.3
HistologicalanalysesofAITLspecimensshoweffacement of the lymph node architecture, particularly in advanced stages.Malignantcells tendtodistributeintointerfollicular regions,andaretypicallypositiveforT-helpercellmarkersand T-cellreceptors(TCRs)alphaandbeta.2,4Immunoblasts,often
positiveforEpstein–Barrvirus(EBV),arefrequentlydispersed inparacortical regions.Thischaracteristiccanbeconfused withReed-Sternbergcells,whichcanleadtoamistaken diag-nosisofHodgkin’slymphoma(HL).TCRgenerearrangements arefoundin70%ofcases.Ontheotherhand,immunoglobulin generearrangementsarefoundinonly10%ofpatientswith AITL.5
There is nostandard treatment forAITL. Consequently, patients may be treated with different drugs, including steroids,immunomodulatorsorbycytotoxicchemotherapy.
http://dx.doi.org/10.1016/j.bjhh.2015.11.002
revbrashematolhemoter.2016;38(1):82–85
83
However,themostcommonlyusedtreatmentmodalityisthe cyclophosphamide,vincristine,doxorubicin andprednisone (CHOP)regimen,associatedornotwithetoposide.This treat-mentistypicallyfollowedbyautologoushematopoieticstem celltransplantation.Furthermore,thenaturalhistoryofAITL ischaracterizedbyseveralrelapses,withafive-yearoverall survivalof30%.6,7
Inthiscasereport,weanalyzedthemainclinical charac-teristicsthatmakeAITLdiagnosisdifficult.AsAITLisarare diseasewithapoorprognosis,anearlyandcorrectdiagnosis isessentialtoimprovesurvivalandqualityoflife.
Case
report
A 56-year-old man with generalized lymphadenomegaly
(neck,abdomen,inguinal,supraclavicularandaxillarregions) cametotheInstitutodoCâncerdoEstadodeSãoPaulofor treatment.Thediseasewasfirstnoticedthreemonths pre-viously.Theinitiallymphnodebiopsy,performedinanother
hospital,suggestednodularsclerosisHL.Itwasdescribedas alymphoidinfiltrateinabackgroundofeosinophils, promi-nentvesselsandlargecells,suggestiveofReed-Sternbergcells (CD45+,CD3−CD20−,CD30+,CD15+,andEBVnegative).
Atadmission,thecomplete bloodcountshowed10.2g/L hemoglobin, 12.4×109/L white blood cell count with
5.4×109/L lymphocytes and 270×109/L platelets.
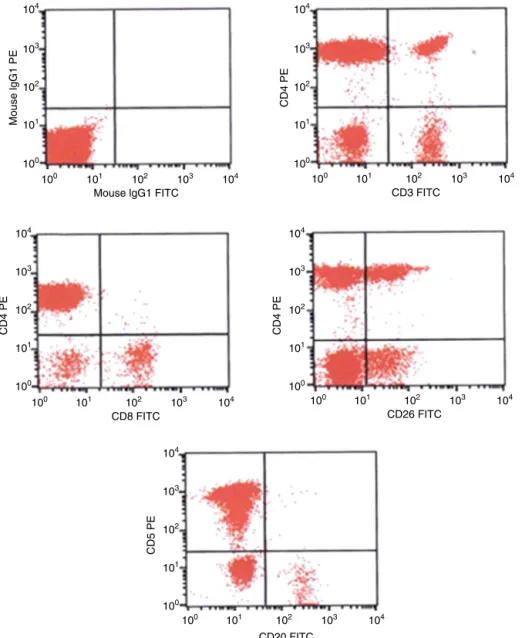
Leuko-cyteimmunophenotypingbyflowcytometryshowed11%of
mCD3−CD5+,CD4bright,CD8−andCD26partiallymphoidT-cells
(Figure1).Furthermore,abonemarrowbiopsydemonstrated absenceoflymphomainfiltration.
Thepatientwassubmittedtoafluorine-18
fluorodeoxyglu-cose positron emission tomography scan that showed
increased uptake of FDG located in superficial and deep lymphnodechains.Theexamalsoindicatedareversalofthe metabolicpatternbetweenliverandspleen.
A review of the histologicalspecimens from the previ-ous biopsy at our center revealed atypical lymphoid cells with expansion in the paracortical zone and a moderate
number of eosinophils. However, we did not repeat the
104
103
102
101
100
Mouse lgG1 PE
Mouse lgG1 FITC
104
103
102
101
100
CD4 PE
104
103
102
101
100
CD4 PE
CD20 FITC 104
103
102
101
100
100 101 102 103 104
CD8 FITC
100 101 102 103 104
CD26 FITC
100 101 102 103 104
CD3 FITC
100 101 102 103 104
100 101 102 103 104
CD5 PE
104
103
102
101
100
CD4 PE
84
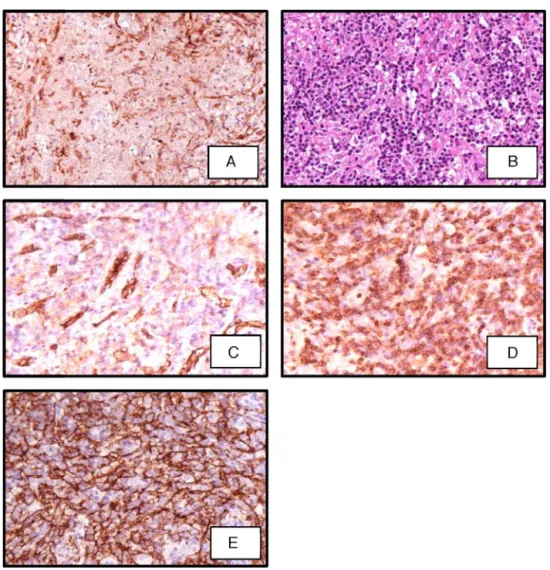
revbrashematolhemoter.2016;38(1):82–85Figure2–Sectionoflymphnodebiopsyshowing(A)immunoperoxidase(IP)CD2showinglymphoidcellsCD2+;(B)
polymorphicinfiltrateincludingeosinophilsandsmalllymphocytes(hematoxylin–eosin400×);(C)IPstainshowing
lymphoidcellsCD10+;(D)IPstainforCD21showingdendriticfollicularcells;(E)IPstainforCD31showingahighquantityof vessels.
immunohistochemicalanalysisinthissamplebecauseitwas verysmall.Therefore, anotherbiopsy wasindicated, and a corebiopsywaschosenbecauseitcouldbedonefaster.The histologicalanalysisofthenewmaterialwascompatiblewith reactivelymphadenopathy.
Inthe next few days, the patient’s condition worsened. Inthemeantime,anotherbiopsywasperformed,thistimea surgicallymphnodebiopsy.However,theimmunochemistry analysiswasincompletewhenthepatient’sconditionbecame aggravated.Astherevisionoftheinitialbiopsywas compati-blewithHL,andduetothequickdeteriorationinthepatient’s performancestatus,ourteamoptedtostarttreatmentforHL withthedoxorubicin,bleomycin,vinblastine,anddacarbazine (ABVD)regimen.
AftertwoABVDcyclesthepatientdevelopedsevere pan-cytopenia.Thetreatmentwasinterruptedduetotoxicity.As itwasnottheexpectedclinicalresponse,thediagnosisofHL wasoncemorequestioned.Atthattime,anewbonemarrow biopsywasmade,andthehistologyshowedthatthebone mar-rowtissuewastotallysubstitutedbyfibrosis.Simultaneously, theresultsofapolymerase chainreaction(PCR) evaluation
ofthe monoclonalrearrangement oftheTCR-gamma gene
becameavailable,andtheywerepositive:thesenewdata sug-gestedamatureT-celllymphoproliferativedisease.
Afewdayslater,theimmunohistochemistryofthe exci-sionallymphnodebiopsywascompletedandthediagnosis of AITL was confirmed (Figure 2). In the meantime, the patient’sclinicalconditiondeteriorateddrastically,soanother appropriate chemotherapeutic scheme was not prescribed. Unfortunately,thepatientdiedinashortperioddueto respi-ratorycomplications.
Discussion
revbrashematolhemoter.2016;38(1):82–85
85
example,AITLsymptomsincludeskinrash,fever,generalized lymphadenopathyandpolyclonalhypergammaglobulinemia, whicharecommon featuresofinfections andautoimmune disorders.3
ThegoldstandardforAITLdiagnosisisexcisionallymph nodebiopsy.However,tosavetimeandtoavoidexposureto invasiveprocedures,manycentersperformacorebiopsyto obtainsamplesforpathologicalanalyses.Unfortunately,the samplesobtainedbycorebiopsy canbeinsufficientto per-formacompleteimmunohistochemicalpanelandensurethe correctdiagnosis.
Previousstudieshaveshownthattheaccuracyofa lym-phomadiagnosisbasedoncorebiopsiesvariesfrom68%to 94%.8Inadequatecorebiopsiesareassociatedwith
misdiag-nosesordelayeddiagnoses.Guptaetal.comparedlymphnode biopsiesacquiredwith eitherfine-needleaspiration or sur-gicalexcision in100patients. Theyfoundthatthe ratesof accuratediagnosesbasedonfine-needleaspirationswere77% inreactivehyperplasia,75%inNHL,and85%inmetastatic carcinoma.Arecentmeta-analysisshowedthatcorebiopsies providedadequatematerialforhistologyin95%ofcases, par-ticularlyinsalivaryglandlesions,butinadequatematerialin 39(2.6%)casesoflymphadenopathiesoftheheadandneck. Indifferentiatingbetweenmalignantlymphomaandreactive lymphnodes,corebiopsiesshowedahighfalse-negativerate andalownegativepredictivevalue(85%).9
Afullhistologicalandimmunohistochemicalanalysisof thelymphnodeisessentialforthe differentiationbetween AITL and other diseases. For example,it can differentiate betweenlargecellswithtwoormorenuclei,whichare
fre-quently observed in AITL tumor microenvironments, and
Reed-Sternbergcells.3,4 Inthepresentcasestudy,thesmall
amount oftissuewasinsufficient toperforman expanded immunohistochemicalpanel,andconsequently,thediagnosis wasincorrect.
Singhet al.demonstrated that17/17 patientswithAITL in the leukemic phase harbored a distinct population of sCD3−/CD4+T-cellsintheperipheralblood.Furthermore,this phenotypewashighlyspecifictoAITLbecauseitwasfound in only 1/40 patients with other T-cell lymphomas in the leukemicphase.Theyshowedthatthisphenotypeprovideda positivepredictivevalueof94%foradiagnosisofAITL.Those authorsconcludedthatimmunophenotypingperipheralblood withflowcytometrymightbeausefulmethodforachieving adifferentialdiagnosisofAITL,eventhoughtheaberrant T-cellpopulationoccursataverylowfrequencyinperipheral blood.10 Therefore, thisassay should bepart oflymphoma
investigations,especiallyininconclusivecases,becauseitcan savetimeandimprovetheaccuracyofdiagnosis.
Inthiscasereport, wedescribeacaseofAITL thatwas erroneouslytreatedasHL.Westatethatthismistaken diag-nosis was mainly a consequence ofan insufficient biopsy sample,whichledtoanincompletehistologicalanalysis.We recommendthat inrefractorycasesacomplete revisionof
the initialbiopsy shouldbeperformedassoonaspossible. Wealsostronglyrecommendthattoobtainanaccurateand precisediagnosis forlymphoma, excisionalbiopsiesshould beperformedinsteadofcorebiopsies.Ancillarystudies,such asperipheralbloodimmunophenotypingandPCRdetection ofTCRrearrangements,areveryimportanttoolstoestablish differentialdiagnoses,andshouldbepartoftheinvestigation toimprovetheaccuracyortoconfirmthediagnosisofAITL.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.FrizzeraG,MoranE,RappaportH.Angioimmunoblastic lymphadenopathywithdysproteinemia.Lancet. 1974;1(1766):1070–3.
2.WHOclassificationoftumorsofhematopoieticandlymphoid tissues.4thed.Lyon:IARCPress;2008.
3.MouradN,MounierN,BrièreJ,RaffouxE,DelmerA,FellerA, etal.Clinical,biologic,andpathologicfeaturesin157patients withangioimmunoblasticT-celllymphomatreatedwithin theGroupd’EtudedesLymphomesdel’Adulte(GELA)trials. Blood.2008;111(9):4463–70.
4.LaforgaJB,GasentJM,VaqueroM.Potentialmisdiagnosisof angioimmunoblasticT-celllymphomawithHodgkin’s lymphoma:acasereport.ActaCytol.2010;545Suppl:840–4.
5.KawanoR,OhshimaK,WakamatsuS,SuzumiyaJ,KikuchiM, TamuraK.Epstein-Barrvirusgenomelevel.T-cellclonality andtheprognosisofangioimmunoblasticT-celllymphoma. Haematologica.2005;90(9):1192–6.
6.KyriakouC,CanalsC,GoldstoneA,CaballeroD,MetznerB, KobbeG,etal.High-dosetherapyandautologousstem-cell transplantationinangioimmunoblasticlymphoma:complete remissionattransplantationisthemajordeterminantof Outcome-LymphomaWorkingPartyoftheEuropeanGroup forBloodandMarrowTransplantation.JClinOncol. 2008;26(2):218–24.
7.FedericoM,RudigerT,BelleiM,NathwaniBN,LuminariS, CoiffierB,etal.Clinicopathologiccharacteristicsof angioimmunoblasticT-celllymphoma:analysisof InternationalPeripheralT-cellLymphomaProject.JClin Oncol.2013;31(2):240–6.
8.Ben-YehudaD,PolliackA,OkonE,ShermanY,FieldsS, LebenshartP,etal.Image-guidedcoreneedlebiopsyin malignantlymphoma:experiencewith100patientsthat suggeststhetechniqueisreliable.JClinOncol.
1996;14(9):2431–4.
9.NovoaE,GürtlerN,ArnouxA,KraftM.Roleofultrasound guidedcore-needlebiopsyintheassessmentofheadand necklesions:ameta-analysisandsystematicreviewof literature.HeadNeck.2012;34(10):1497–503.
10.SinghA,SchabathR,RateiR,StrouxA,KlemkeCD,NebeT, etal.PeripheralbloodsCD3(−)CD4(+)T-cells:auseful