w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
A
new
musculoskeletal
ultrasound
scoring
system
(US10)
of
the
hands
and
wrist
joints
for
evaluation
of
early
rheumatoid
arthritis
patients
Karine
R.
Luz
a,
Marcelo
M.
Pinheiro
a,
Giovanna
S.
Petterle
a,
Marla
F.
dos
Santos
a,
Artur
R.C.
Fernandes
b,
Jamil
Natour
a,∗,
Rita
N.V.
Furtado
aaDivisãodeReumatologia,UniversidadeFederaldeSãoPaulo,SãoPaulo,SP,Brazil bDepartamentodeRadiologia,UniversidadeFederaldeSãoPaulo,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received29September2014 Accepted25March2016 Availableonline6August2016
Keywords:
Earlyrheumatoidarthritis Hand
Ultrasound Score
a
b
s
t
r
a
c
t
Objective:Toproposeanovelultrasoundscoringsystemforhandandwristjoints(US10)for evaluationofpatientswithearlyrheumatoidarthritis(RA)andtocorrelatetheUS10with clinical,laboratoryandfunctionalvariables.
Methods:Forty-eightearlyRApatientsunderwentclinicalandlaboratoryevaluations as wellasblindedultrasound(US)examinationsatbaseline,three,sixand12months.The proposedUS10systeminvolvedtheassessmentofthewrist,secondandthird metacar-pophalangealandproximalinterphalangealjoints.Thescoreconsistedofinflammation parameters(synovialproliferation[SP],powerDoppler[PD]andtenosynovitis[TN])andjoint damageparameters(boneerosion[BE]andcartilagedamage[CD]).SP,PD,BEandCDwere scoredqualitatively(0–1)andsemi-quantitatively(grades0–3).Tenosynovitiswasscoredas presence/absence.Theevaluationalsoinvolvedthe28-JointDiseaseActivityScore(DAS28), HealthAssessmentQuestionnaire(HAQ)andC-reactiveproteinlevel(CRP).
Results:Mean duration of symptoms was 7.58±3.59 months. Significant correlations (p<0.05)werefoundbetweeninflammationparametersandCRPatbaselineandbetween thechangesinthesevariablesthroughoutthestudy.Significantcorrelations(p<0.05)were foundbetweenDAS28scoreandbothPDandTNatbaselineandbetweenthechangesin DAS28scoreandbothSPandTNthroughoutthefollowup.Moreover,significant correla-tionswerefoundbetweenthechangesininflammationparameterscoresandHAQscore throughoutthefollowup.
∗ Correspondingauthor.
E-mail:jnatour@unifesp.br(J.Natour).
http://dx.doi.org/10.1016/j.rbre.2016.07.006
Conclusion: TheproposedUS10scoringsystemprovedtobeausefultoolformonitoring inflammationandjointdamageinearlyRApatients,demonstratingsignificantcorrelations withlongitudinalchangesindiseaseactivityandfunctionalstatus.
©2016ElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Novo
sistema
de
escore
ultrassonográfico
(US10)
musculoesquelético
das
articulac¸ões
das
mãos
e
punho
para
avaliac¸ão
de
pacientes
com
artrite
reumatoide
em
fase
inicial
Palavras-chave:
Artritereumatoideemfase inicial
Mão Ultrassom Escore
r
e
s
u
m
o
Objetivo: Proporumnovosistemadeescoreultrassonográficodasarticulac¸õesdamãoe punho(US10)paraaavaliac¸ãodepacientescomartritereumatoide(AR)ecorrelacionaro US10comvariáveisclínicas,laboratoriaisefuncionais.
Métodos: Foramsubmetidos48pacientescomARemfaseinicialaavaliac¸õesclínicase lab-oratoriais,bemcomoaexamescegosdeultrassom(US)noiníciodoestudoecom3,6e 12 meses.Osistema US10propostoenvolveua avaliac¸ãodopunho edas articulac¸ões metacarpofalângicaseinterfalângicasproximaisdosegundoeterceirodígitos.Oescore consistiu em parâmetros inflamatórios(proliferac¸ão sinovial[PS], Power Doppler [PD] e tenossinovite [TN]) e parâmetros de danos articulares (erosão óssea [EO] e danos na cartilagem[DC]).PS,PD,EOeDCforampontuadosqualitativamente(0a1)e semiquantita-tivamente(graus0a3).Atenossinovitefoipontuadacomopresenc¸a/ausência.Aavaliac¸ão envolveutambémoescore28-JointDiseaseActivity(DAS28),oHealthAssessmentQuestionnaire
(HAQ)eoníveldeproteínaC-reativa(PCR).
Resultados: Adurac¸ãomédia dossintomasfoi de7,58±3,59meses.Foramencontradas correlac¸õesestatisticamentesignificativas(p<0,05)entreosparâmetrosdeinflamac¸ãoea PCRnoiníciodoestudoeentreasmudanc¸asnessasvariáveisaolongodoestudo.Foram encontradastambémcorrelac¸õessignificativas(p<0,05)entreoescoreDAS28eaPDeTN noiníciodoestudoeentreasmudanc¸asnoescoreDAS28ePSeTNemtodooseguimento. Alémdisso,foramencontradascorrelac¸õessignificativasentreasmudanc¸asnoescoredos parâmetrosdeinflamac¸ãoenoescoreHAQaolongodoseguimento.
Conclusão: OsistemadeescoreUS10propostoprovouserumaferramentaútilpara mon-itorarainflamac¸ãoeodanoarticularem pacientescomARemfaseinicial,demonstra correlac¸õessignificativascomasalterac¸õeslongitudinaisnaatividadedadoenc¸aenoestado funcional.
©2016ElsevierEditoraLtda.Este ´eumartigoOpenAccesssobumalicenc¸aCC BY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
In recent years, musculoskeletalultrasound (US)has been employedfor monitoring patients with rheumatoid arthri-tis (RA) with regard to both disease activity and joint damage.1–6 Disease activity is evaluated using gray-scale and Power Doppler (PD) synovitis and tenosynovitis.4,5,7,8 However, there is no consensus on the US evaluation of synovitis (synovial proliferation and PD) in the joints, as scoring is performed with either a binary variable (pres-ence/absence of synovitis) or a semi-quantitative variable (usuallyusingascalefrom0[absenceofsynovitis]to3[severe synovitis]).9–14
UScanalsobeusedtoassessjointdamageandhasproven to be sensitive to the detection ofbone erosion, which is
importanttothediagnosisandevaluationofRA,especiallyin smalljointsofthehandsandfeet.6,10Indeed,USisbetterable todetecterosionsinthemetacarpophalangealjoints(MCPs) inpatientswithearlyRAthanradiography.6 Likesynovitis, bone erosion can be evaluated using a semi-quantitative scoring system, with excellent interobserver agreement (ICC=0.78andk=0.68).10
Anumber ofstudies haveevaluateddifferent simplified scoresandreportsatisfactorycorrelationswithclinical dis-easeactivity indices.15–18 Thejoints ofthehands(proximal interphalangealPIPsandMCPs)andwristarethemostaffected
joints in disease-modifying antirheumatic drugs (DMARD)-naïvepatientswithearlyRAmaybeusefultoclinicalfollow-up andformonitoringtherapy.
Thus,theaimofthe presentstudy wastoinvestigate a novelUSscoringsystemfortheevaluationofinflammation andjointdamageinthehandandwristsjoints,denominated theUS10,andcorrelateUSinflammationand jointdamage parameters with clinical, laboratory, functional and radio-graphicfindingsinpatientswithearlyRAovera12months offollowup.
Patients
and
methods
Patients
A prospective cohort study was conducted involving 48 consecutive patients with early RA, with symptom dura-tion for more than six weeks and less than one year sinceonset. Theindividuals were recruited from Rheuma-tologyOutpatientClinicsoftheUniversidadeFederaldeSão Paulo.ThisstudyreceivedapprovalfromtheResearchEthics Committee.
Theinclusioncriteriawerethefollowing:diagnosisofRA basedonthe1987and2010classificationcriteriaofthe Amer-icanCollegeofRheumatology(ACR)oracutoffpointof≥8.0 onpredictivevalidationLeidenmodelscore.23,24 TheLeiden scoreconsistsofnineclinicalandlaboratoryvariables includ-ingage,sex,locationofjointsymptoms,morningstiffness, tenderandswollenjointcount,CRPlevelsandpresenceofRF andanti-CCPantibody.Eachitemhasavalue,andthe varia-tionof0–14score.ApatientisclassifiedasearlyRAifpresents ascore≥8.25
Theexclusioncriteriawerethefollowing:previous treat-mentwith DMARDs,use oforalglucocorticoid>10mg/din the previous three weeksor a parenteral glucocorticoid in thepreviousfourweeks,serumaspartateaminotransferase oralanine aminotransferaselevel >3times the upperlimit ofnormal,bonemarrowhypoplasia,overlapwithanyother collagen disease, suspicion of lymphoproliferative disease, positiveserologyforhepatitisBorCandpregnancy.
Allpatientsunderwentclinical,laboratoryandUS evalua-tionsatbaseline,three,sixand12months.Atightlycontrolled therapeutic protocol was used for all patients by a single rheumatologistwhowas blindedtotheUS evaluation.The patientsbeganwithmethotrexate(MTX)15mg/week,which wasincreasedto25mg/weekinthefirstthreemonths. Subse-quentstepsforpatientswithaninsufficientresponse(DAS28 score>3.2andevaluator-basedglobalassessmentofdisease activity score>4.0 [0–10cm]) were leflunomide with MTX 15mg/week,leflunomideandMTX25mg/week,adalimumab andMTX15mg/weekand,finally,MTX15mg/weekwitha sec-ondbiologicagent.
Clinicalassessment
The patients were clinically evaluated during each visit. Twenty-eight joints (bilateral PIPs, MCPs, wrists, elbows, shoulders and knees) were clinically assessedfor swelling and tenderness. The following instruments were also
employed:Evaluatorbasedglobalassessmentofdisease activ-ity (0–10cm); patient based global assessment of disease activity(0–10cm);Brazilianversionofthefunctionalsubscale of the Stanford Health AssessmentQuestionnaire (HAQ)26; andthe28-JointDiseaseActivityScore(DAS28).
Laboratoryevaluation
ThedosageofC-reactive protein(CRP)level (mg/dliter)and erythrocytesedimentationrate(ESR)(mm/hour)were deter-mined ateach visit. IgMrheumatoid factor and anti-cyclic citrullinated peptide(anti-CCP)antibodieswere assessedat baseline.
Ultrasoundassessment
TheUSexaminationwasperformedbyatrained rheumatol-ogistwitheightyearsofexperienceinUSwhowasblinded toallotherstudyfindings.USexaminationswereperformed usingaMyLab60(Esaote,Biomedica–Genoa,Italy),equipped withabroadbandlinearprobewithfrequencyrangingfrom6 to18MHz.
Systematicmultiplanargray-scaleUS(GSUS)andPD exam-inationswereperformedon10joints(wrist,MCP2,MCP3,PIP2 andPIP3inbothhands)inastandardizedmannerbasedon theguidelinesoftheEuropeanLeagueAgainstRheumatism.27 Alljointregionswereassessedusinginflammationandjoint damageparameters.
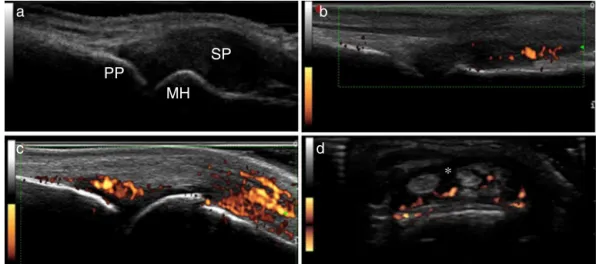
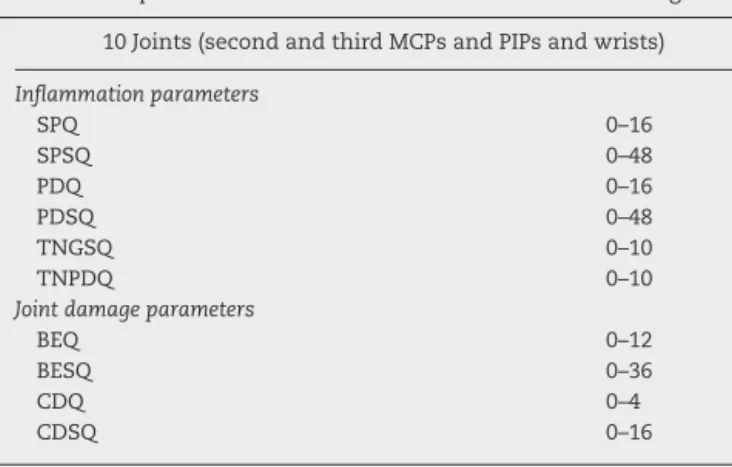
A. Inflammationparameters(Fig.1): 1. Synovialproliferation(SP):
SynovitisbyGSUS–synovialproliferation,definedas an abnormalhypoechoicintra-articulartissue thatis non-displaceableandpoorlycompressiblevisualizedin longitudinalandtransversalplanes28;Thefollowing20 synovialsitesin10jointswereincluded:wrist(dorsal carpalandulnarcarpalrecess);secondandthirdMCP (dorsalside,palmarside);secondandthirdPIP(palmar side).
Synovialproliferationwasanalyzedinallsites,as fol-lows:
- Semi-quantitative evaluation (SPSQ) – Grade 0 (absence), Grade1 (smallhypoechoic/anechoic line beneathjointcapsule),Grade2(jointcapsuleelevated paralleltojointarea)andGrade3(strongdistension ofjointcapsule)10,14;
- Qualitativeevaluation(SPQ)–binaryevaluation–0 (absent)or1(present,ifGrade2or3semi-quantitative scores).
2. Synovialbloodflow:
SynovialbloodflowwasevaluatedbyPDineachofthe intra-articularsynovialsites.PDsettingswere standard-izedwithapulserepetitionfrequencyof750Hzanda color-modefrequencyof12MHz.Wallfiltersweresetat thelowestvalue,whilecolorgainwasincreasedtothe highestvalue,notgeneratingPDsignalsunderthebone cortex.
a
PP
SP
MH
b
d
c
∗
Fig.1–InflammationparametersofUS10.(A)Synovialproliferationgrade3;(B)synovitisbypowerDopplergrade2;(C) synovitisbypowerDopplergrade3;(D)tenosynovitisbypowerDoppler.PP,proximalphalanx;MH,metacarpalhead;SP, synovialproliferation;*,tenosynovitis.
- Semi-quantitative(PDSQ)–Grade0(noflowin syn-ovium), Grade 1 (single vessel signals); Grade 2 (confluentvesselsignalsinlessthanhalftheareaof thesynovium);Grade3(vesselsignalsinmorethan halftheareaofthesynovium)10;
- Qualitative(PDQ)–binaryevaluation–0(absent)or1 (present,ifGrade1semi-quantitativescore). 3. Tenosynovitis
Definedasahypoechoicoranechoicthickenedtissue withorwithoutfluidwithinthetendonsheath28; Thefollowingtendonswereevaluated:extensor digito-rumcommunis;extensorcarpiulnaris;flexordigitorum communis,secondandthirdflexortendons.
TenosynovitiswasevaluatedandgradedonaGSUSand PDUSqualitativescore:
- Qualitative(TNGSQ)–binaryevaluation–0(absent) or1(present);
- Qualitative(TNPDQ)–binaryevaluation–0(absent) or1(present).
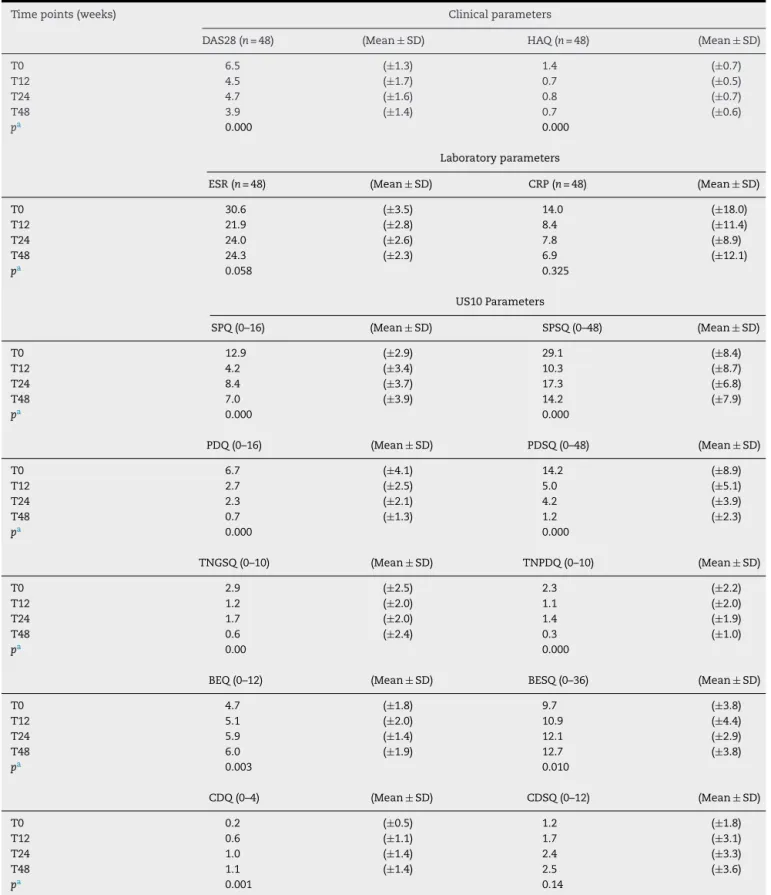
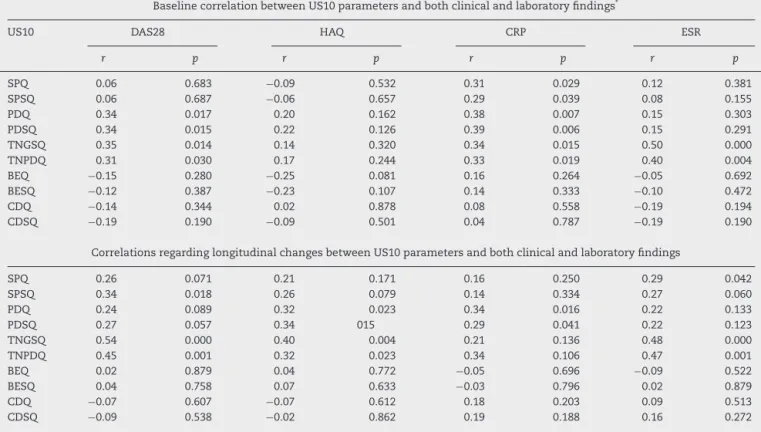
B. Jointdamageparameters(Fig.2): 1. Boneerosion
Erosionwasdefinedasaninterruptionofthebone sur-faceontwoperpendicularplanes.28
Thelocationofeacherosionwasrecordedbasedonthe boneinvolved,asfollows:
- Dorsalquadrantofsecondandthirdmetacarpalhead - Lateralquadrantofsecondmetacarpalhead
- Dorsalquadrantofsecondandthirdphalanx - Ulnarstyloidprocess
Boneerosionsweregradedonaqualitativeand semi-quantitativescore:
- Semi-quantitative (BESQ) – Grade 0 (regular bone surface), Grade 1 (irregular bone surface without formation of defect seen on two planes), Grade 2
a
b
d
c
PP
PP
PP
MH
MH
MH
USP
Table1–US10:Scorerangeforeachultrasoundhand parameter.
Ultrasoundparameters–US10 Scorerange
10Joints(secondandthirdMCPsandPIPsandwrists)
Inflammationparameters
SPQ 0–16
SPSQ 0–48
PDQ 0–16
PDSQ 0–48
TNGSQ 0–10
TNPDQ 0–10
Jointdamageparameters
BEQ 0–12
BESQ 0–36
CDQ 0–4
CDSQ 0–16
US10,ultrasoundscoreofhandjointsandwrists;MCP, metacar-pophalangealjoint;PIP,proximalinterphalangealjont;SPQ, quali-tativesynovialproliferation;SPSQ,semi-quantitativesynovial pro-liferation;PDQ,qualitativepowerDoppler;PDSQ,semi-quantitative power Doppler; TNGSQ, qualitative gray-scale tenosynovitis; TNPDQ,qualitativepowerDopplertenosynovitis;BEQ,qualitative boneerosion;BESQ,semi-quantitativeboneerosion;CDQ, qualita-tivecartilagedamage;CDSQ,semi-quantitativecartilagedamage.
(formation of defect on bone surface seen on two planes)andGrade3(bonedefectcausingextensive bonedestruction)10;
- Qualitative(BEQ)–binaryevaluation–0(absent)or1 (present,ifGrade2or3semi-quantitativescores). 2. Cartilagedamage
USexaminationswerefocusedontheassessmentofthe hyalinecartilageinthedorsalviewofthesecondand thirdmetacarpalheads.Normalfeaturesofthehyaline cartilageatthemetacarpalheadincludedtwo hyperec-hoicsharp,regularandcontinuousmarginsdelimiting ahomogenousanechoicband.29,30
Cartilage damagewas evaluated using the following semi-quantitativeandqualitativescoringsystem: - Semi-quantitative(CDSQ)–Grade0(normalhyaline
cartilage); Grade1 (lossofsharpness ofsuperficial marginofhyalinecartilage);Grade2(partialthickness defectofcartilagelayer);Grade3(fullthicknessdefect ofcartilagelayerwithnormalsubchondralbone pro-file); Grade4 (complete loss ofcartilage layer and subchondralboneinvolvement).31,32
- Qualitative(CDQ)–binaryevaluation–0(absent)or1 (present,ifGrade2or3semi-quantitativescores). TheUS10scoringsystemanalyzed10sonographic param-eters,andeachparameterwassubdividedintoqualitative andsemi-quantitativegrades.Theparameterswere ana-lyzed separately, as indicated in Table 1. These 10 parameters and 10-joints constituted the US10 system, withthesumofallscores(Table1).
Interobserverreliability
InterobserverreliabilitybetweentwoUSoperatorswas evalu-atedonrecordedimagesfrom20randomlychosenpatientsfor
theinflammationandjointdamageparametersofthejoints includedintheUS10score.ThecapturedimagesofeachUS10 item of20 patientswitha totalof200images were evalu-ated.Theevaluationoftheultrasoundimageswasperformed byarheumatologist,withfiveyearsofexperiencein muscu-loskeletalultrasound.
Radiographicevaluation
PosteroanteriorX-raysofthepatients’handswere recorded atbaselineandafter12months.Theimageswereanalyzedat theendofthestudybyaradiologistwhowasblindedtothe otherevaluations.TheevaluationofhandX-rayswasdoneby aradiologistwith30yearsofexperiencewhowasblindtothe clinicalfeatures,ultrasoundresultsandidentificationofthe patient.Intheradiographicevaluationitwasconsideredonly boneerosion.Thepresenceoferosionswasevaluatedusing thescoresproposedbyvanderHeijdeetal.33Thecorrelation betweennarrowingofjointspaceonX-raysandjointcartilage damageonultrasoundwasnotanalyzed.
Statisticalanalysis
StatisticalanalysiswasperformedwiththeaidoftheSPSS program, version 17.0 (SPSS, Chicago, IL, USA). Data are expressed as mean±standard deviation. ANOVA was per-formedtocompare numericalvariables repeatedovertime. Correlations betweenchangesinthe differentexamination modalities(clinical,laboratoryandUS)throughoutthe follow-upperiodwereevaluatedbytwo-tailedSpearman’scorrelation coefficients. Inter- and intra-reader agreement was calcu-lated using kappacoefficients betweenreaders. Thekappa coefficientsweredividedasfollows:<0.0=poor,0–0.20=slight, 0.21–0.40=fair,0.4–0.60=moderate,0.6–0.80=substantialand 0.81–1.0=almostperfectagreement.34Comparisonsbetween X-ray and US regarding bone erosion were made using the chi-square test, adjusted by the McNemar method. The statistical significance level was set to 5% (p<0.05). The data were analyzed using the intention-to-treat principle.
Results
Patientcharacteristics
Table2–Baselinedemographicparameters,disease relatedvariablesin48RA.
RApatients
(n=48)
Age,years(mean±SD) 47.7±11.6
Gender(women/men) 48/0
Diseaseduration,months(mean±SD) 7.5±3.5
Rheumatoidfactor(positive)(%) 20(41.7%)
Anti-CCP(positive)(%) 21(43.8%)
1987ACRcriteria(%) 30(62.5%)
2010ACRcriteria(%) 48(100%)
LeidenModelScore(%) 35(72.9%)
RA,rheumatoidarthritis;ACR,AmericanCollegeofRheumatology; SD,standarddeviation.
Clinical,laboratoryandUS10parameters
Table3displaystheUS,clinical andlaboratorydata.A sig-nificant reduction in mean DAS28 score was found over 12 months (6.5–3.9; p<0.05). The mean HAQ score also decreasedsignificantly(1.4–0.7;p<0.05).Nosignificant differ-ences were found inmean CRP (14.0–6.9mg/dliter; p>0.05) or ESR(30.6–24.3mm/h;p>0.05) levels over 12 months.All US10inflammationparametersdecreasedsignificantlyover the course ofthe year. Mean SPQ and SPQSQ scores were respectively12.9and 29.1atbaselineanddecreased signif-icantlyafter12months(7.0and14.2,respectively)(p<0.05). MeanbaselinePDQandPDSQscoreswere6.7and14.2, respec-tively,and decreasedsignificantlyand after12months(0.7 and 1.2, respectively) (p<0.05). Mean TNGSQ and TNPDQ scores reduced respectively from 2.9 and 2.3 at baseline to 0.6 and 0.3 after 12 months (p<0.05). Mean qualitative andsemi-quantitativeboneerosionscoresincreased signif-icantly over 12 months (4.7–6.0 and 9.7–12.7, respectively) (p<0.05).Asignificantincreaseinthemeanqualitative carti-lagedamagescoreoccurredover12months(0.2–1.1;p<0.05). Nosignificant difference inthe mean semi-qualitative car-tilage damage score occurred over 12 months (1.2–2.5;
p=0.14).
BaselineandlongitudinalcorrelationbetweenUS10
parameters
Atbaseline,allUS10inflammationparameterscores demon-strated significant correlations (p<0.05) with each other (r
variation between 0.33 and 0.95). A significant correlation (p<0.05) was found between changes in the SPSQ score and changes in both the PDQ (r=0.43) and PDSQ (r=0.42) scoresandTNGSQ(r=0.47)andTNPDQ(r=0.40)scoresover 12months.
BaselineandlongitudinalcorrelationsbetweenUS10,
clinicalandlaboratoryparameters
All US inflammation parameters (SPQ, SPSQ, PDQ, PDSQ, TNGSQ and TNPDQ) demonstrated significant correlations withCRPlevelsatbaseline(r=0.31,r=0.29,r=0.38,r=0.39,
r=0.34 and r=0.33, respectively)(p<0.05). All PD(PDQ and PDSQ) and tenosynovitis (TNGSQ and TNPDQ) scores were significantlycorrelated(p<0.05)withDAS28(r=0.34,r=0.34,
r=0.35, r=0.31, respectively). The GS and PD tenosynovi-tisscorescorrelatedsignificantly(p<0.05)withESR(r=0.50,
r=0.40,respectively). Nocorrelationwas observedbetween the change of the qualitative score of synovial prolifera-tion (SPQ),andDAS 28overtime.However itwasobserved correlation between the semi-quantitative score synovial proliferation (SPSQ) and DAS28 scores. Changes in PD and tenosynovitis scores were significantlycorrelated with changes in HAQ over 12 months (r=0.32, r=0.34, r=0.40,
r=0.32,respectivelywithp<0.05).ChangesinPDscoresalso correlatedwithchangesinCRP(r=0.34,r=0.29,respectively). Changes in qualitative synovial proliferation and tenosyn-ovitis scores (TNGSQ and TNPDQ) correlated with changes in ESR (r=0.29, r=0.48, r=0.49, respectively with p<0.05). There was nocorrelation betweensonographic parameters ofjointdamage(cartilageanderosion)andclinicaland lab-oratoryvariables.Thecorrelationsbetweenthesonographic parametersandclinicalandlaboratoryvariablesareshownin
Table4.
Inter-observerreliabilityregardingUS10parameters
Meankappavaluesforthequalitativeandsemi-quantitative synovial proliferation scores were 0.49 and 0.21, respec-tively (p<0.05). Meankappa valuesforPDQ, PDSQ, TNGSQ and TNPDQ scores on stored images were 0.49, 0.56, 0.55 and 0.32, respectively (p<0.05). Mean kappa values for qualitative and semi-quantitative bone erosion scores were 0.42and0.47,respectively(p<0.05).Substantial agree-ment betweenobservers wasfoundforthe qualitativeand semi-quantitativecartilagedamagescores(k=0.79and0.82) (p<0.05).
Comparisonofconventionalradiographyandultrasound
forboneerosionatbaselineandafter12months
In 480 sites analyzed at baseline, radiography found ero-sionsin54sites(11.3%)and USfounderosions in150sites (31.3%).USdetected2.81-foldmoreboneerosionsthan con-ventional radiography in the 10 sites examined (p<0.001). After12 months,US found171(39.80%) boneerosions and radiographyfound44(10.20%)erosionsinthe430sites exam-ined. The US detected 3.88-fold more bone erosions than conventional radiographyinthe 10sitesexamined after48 weeks of evaluation. Erosions were evaluated by the two methods described in the same places and in the same patients.
Discussion
Table3–Clinical,laboratoryandUS10scoresovertimeinrelationtobaselineevaluation.
Timepoints(weeks) Clinicalparameters
DAS28(n=48) (Mean±SD) HAQ(n=48) (Mean±SD)
T0 6.5 (±1.3) 1.4 (±0.7)
T12 4.5 (±1.7) 0.7 (±0.5)
T24 4.7 (±1.6) 0.8 (±0.7)
T48 3.9 (±1.4) 0.7 (±0.6)
pa 0.000 0.000
Laboratoryparameters
ESR(n=48) (Mean±SD) CRP(n=48) (Mean±SD)
T0 30.6 (±3.5) 14.0 (±18.0)
T12 21.9 (±2.8) 8.4 (±11.4)
T24 24.0 (±2.6) 7.8 (±8.9)
T48 24.3 (±2.3) 6.9 (±12.1)
pa 0.058 0.325
US10Parameters
SPQ(0–16) (Mean±SD) SPSQ(0–48) (Mean±SD)
T0 12.9 (±2.9) 29.1 (±8.4)
T12 4.2 (±3.4) 10.3 (±8.7)
T24 8.4 (±3.7) 17.3 (±6.8)
T48 7.0 (±3.9) 14.2 (±7.9)
pa 0.000 0.000
PDQ(0–16) (Mean±SD) PDSQ(0–48) (Mean±SD)
T0 6.7 (±4.1) 14.2 (±8.9)
T12 2.7 (±2.5) 5.0 (±5.1)
T24 2.3 (±2.1) 4.2 (±3.9)
T48 0.7 (±1.3) 1.2 (±2.3)
pa 0.000 0.000
TNGSQ(0–10) (Mean±SD) TNPDQ(0–10) (Mean±SD)
T0 2.9 (±2.5) 2.3 (±2.2)
T12 1.2 (±2.0) 1.1 (±2.0)
T24 1.7 (±2.0) 1.4 (±1.9)
T48 0.6 (±2.4) 0.3 (±1.0)
pa 0.00 0.000
BEQ(0–12) (Mean±SD) BESQ(0–36) (Mean±SD)
T0 4.7 (±1.8) 9.7 (±3.8)
T12 5.1 (±2.0) 10.9 (±4.4)
T24 5.9 (±1.4) 12.1 (±2.9)
T48 6.0 (±1.9) 12.7 (±3.8)
pa 0.003 0.010
CDQ(0–4) (Mean±SD) CDSQ(0–12) (Mean±SD)
T0 0.2 (±0.5) 1.2 (±1.8)
T12 0.6 (±1.1) 1.7 (±3.1)
T24 1.0 (±1.4) 2.4 (±3.3)
T48 1.1 (±1.4) 2.5 (±3.6)
pa 0.001 0.14
SD,standarddeviation;HAQ,health assessmentquestionnaire; ESR,erythrocytesedimentationrate (mm/hour);CRP,C-reactive protein (mg/dliter);SPQ,qualitativesynovialproliferation;SPSQ,semi-quantitativesynovialproliferation;PDQ,qualitativepowerDoppler;PDSQ, semi-quantitativepowerDoppler;TNGS,qualitativegray-scaletenosynovitis;TNPDQ,qualitativepowerDopplertenosynovitis;BEQ,qualitativebone erosion;BESQ,semi-quantitativeboneerosion;CDQ,qualitativecartilagedamage;CDSQ,semi-quantitativecartilagedamage.
Table4–BaselineandlongitudinalcorrelationsbetweenUS10parametersandbothclinicalandlaboratoryfindings.
BaselinecorrelationbetweenUS10parametersandbothclinicalandlaboratoryfindings*
US10 DAS28 HAQ CRP ESR
r p r p r p r p
SPQ 0.06 0.683 −0.09 0.532 0.31 0.029 0.12 0.381
SPSQ 0.06 0.687 −0.06 0.657 0.29 0.039 0.08 0.155
PDQ 0.34 0.017 0.20 0.162 0.38 0.007 0.15 0.303
PDSQ 0.34 0.015 0.22 0.126 0.39 0.006 0.15 0.291
TNGSQ 0.35 0.014 0.14 0.320 0.34 0.015 0.50 0.000
TNPDQ 0.31 0.030 0.17 0.244 0.33 0.019 0.40 0.004
BEQ −0.15 0.280 −0.25 0.081 0.16 0.264 −0.05 0.692
BESQ −0.12 0.387 −0.23 0.107 0.14 0.333 −0.10 0.472
CDQ −0.14 0.344 0.02 0.878 0.08 0.558 −0.19 0.194
CDSQ −0.19 0.190 −0.09 0.501 0.04 0.787 −0.19 0.190
CorrelationsregardinglongitudinalchangesbetweenUS10parametersandbothclinicalandlaboratoryfindings
SPQ 0.26 0.071 0.21 0.171 0.16 0.250 0.29 0.042
SPSQ 0.34 0.018 0.26 0.079 0.14 0.334 0.27 0.060
PDQ 0.24 0.089 0.32 0.023 0.34 0.016 0.22 0.133
PDSQ 0.27 0.057 0.34 015 0.29 0.041 0.22 0.123
TNGSQ 0.54 0.000 0.40 0.004 0.21 0.136 0.48 0.000
TNPDQ 0.45 0.001 0.32 0.023 0.34 0.106 0.47 0.001
BEQ 0.02 0.879 0.04 0.772 −0.05 0.696 −0.09 0.522
BESQ 0.04 0.758 0.07 0.633 −0.03 0.796 0.02 0.879
CDQ −0.07 0.607 −0.07 0.612 0.18 0.203 0.09 0.513
CDSQ −0.09 0.538 −0.02 0.862 0.19 0.188 0.16 0.272
HAQ,healthassessmentquestionnaire;ESR,erythrocytesedimentationrate(mm/h);CRP,C-reactiveprotein(mg/dliter);SPQ,qualitativesynovial proliferation;SPSQ,semi-quantitativesynovialproliferation;PDQ,qualitativepowerDoppler;PDSQ,semi-quantitativepowerDoppler;TNGSQ, qualitativegray-scaletenosynovitis;TNPDQ,qualitativepowerDopplertenosynovitis;BEQ,qualitativeboneerosion;BESQ,semi-quantitative boneerosion;CDQ,qualitativecartilagedamage;CDSQ,semi-quantitativecartilagedamage;r,Spearman’scorrelation.
systemsbasedonthesejointsandhavedemonstrated reliabil-ityandcorrelationswithclinicalandlaboratoryfindings.15–18 TheUS10iscomposedofinflammatoryandjointdamage parameters.Qualitative(binary)andsemi-quantitative analy-seswereusedfortheevaluationofsynovialproliferation,PD andjoint damage.Aqualitativescore issimplerand faster forclinicalpractice.However,accordingtoOMERACT,theuse ofa semi-quantitativescoring system offersgreater sensi-tivity forthe assessmentofchangesthroughouttreatment anddemonstratesreproducibilityindifferentstudies.35Inthe presentstudy,tenosynovitiswasalsoevaluatedwitha qual-itativescoreingrayscaleandPD.Onlyaqualitativeanalysis wasperformed,sincethereisnosemi-quantitativescoreyet validatedforthisparameter.Thus,theUS10isthefirstglobal USscoringsystemfortheprospectiveanalysisofchangesin inflammationandjointdamageinpatientswithearlyARwith noprioruseofDMARDs.
TwostudieshaveevaluatedUSinflammatorychangesin patientswithearlyRAandnoprioruseofDMARDs.Thefirst investigatedthesensitivityandpredictivevalueofPDchanges in 28 joints regarding clinical, laboratory and radiological outcomesoveraone-yearperiod.36However,nostatistically significant changeinPD wasfoundand therewas no pre-determinedtreatmentstrategyappliedtothepatients,unlike thepresentstudy.Thesecondstudyinvestigatedsynovial pro-liferationandPDin44jointsinpatientswithearlyRAwho
achievedclinicalremissionafterthesametreatment proto-col.However,nochangeinUSinflammatoryparameterswas describedwithtreatmentovertimeincomparisontothe base-lineevaluation.37Moreover,neitherofthestudiescitedused a globalUS scoringsystemor investigatedthe presenceof tenosynovitis.
PreviousstudieshaveassessedUSchangesovertimeusing aglobal scoringsystemonpatientswithestablishedRAor other chronicinflammatoryjointdiseases.15–18 Asimplified 12-joint scoring system proposed by Naredo et al. (2008) was applied to patients withRA withmean disease dura-tion of 111 months.15 Dougados et al. (2010) investigated different US scoringsystems forsynovial proliferation and PD in 20,28 and 38 joints ofpatients with RAwith mean disease duration of 10 years17 and found changes in the inflammatoryparametersovertimeinrelationtothebaseline evaluation(asinthepresentstudy),butdidnotinvestigate tenosynovitis.Backhausetal.(2009)employedaseven-joint ultrasoundscoretofollowupchangesindifferent inflamma-toryjointdiseases(RA,psoriaticarthritis,spondyloarthritis) andfoundstatisticallysignificantimprovementsinsynovitis parameters (synovialproliferationand PD)and tenosynovi-tisafterthreeandsixmonthsincomparisontothebaseline evaluation.16
significant improvement over a 48-week period. A recent study38 reportsthatthesumofgray-scaleandPDscoresof thetendonsofthelongflexorsofthefingersandulnar exten-sorofthecarpuswassensitivetochange,withareductionin scoresafter12monthsinpatientswithRAusingadalimumab. However,unlikethepresentstudy,therewasnoassessment of the tendons of the digitorum communis flexors of the fingers and the patients had long disease duration. There isevidence that patients in the early stage of the disease experiencegreatertenosynovitis,especiallyinthedigitorum communisflexors.7,8
Thisstudyshowedinadditiontothecorrelationbetween tendonscoreanddiseaseactivity,acorrelationbetweenthe improvements of tendon score with the improvement of patient’sfunction,analyzedbyHAQ.Thus,onemaypostulate thatthetendonsofthehandsandwristsshouldbeincluded inthefollowupofpatientswithRA,asdemonstratedbythe presentUSfindings.
TheonlypreviousUSscoringsystemtoevaluatebone ero-sionswastheseven-jointsystemproposedbyBackhausetal. (2009),which revealednochanges overasix-month period (unlikeinthepresentstudy).16However,asmentionedabove, theauthors evaluatedpatientswithdifferentinflammatory jointdiseases,includingestablishedRA,ratherthanpatients withearlyRA.
Over the 48-week period, the change in the semi-quantitative synovial proliferation score was moderately correlatedwithchangesinPDandtenosynovitisandchanges intheselattertwovariableswerecorrelatedwitheachother. Thesefindingslendsupporttothehypothesisthata reduc-tion occurs in the entire intra-articular and peri-articular inflammatory process following the establishment of ade-quate treatment and underscores the importance of the assessmentoftenosynovitisasaUSmeasureinpatientswith earlyRA.
The US10 proved to be correlated with disease activity parameters (CRPand DAS28). These correlations have also been demonstrated in other US scoring systems.12,15–18,36 Naredoetal.(2005)foundastrongcorrelationbetweenCRP andthenumberofjointswithsynovialproliferationandPD signalsin60jointsinpatientswithestablishedRA.Thesame authorsfoundmoderatecorrelationsbetweensynovial prolif-erationandbothCRP(r=0.33)andtheDAS28(r=0.43)aswell asbetweenPDandbothCRP(r=0.33)andDAS28(r=0.48),in28 jointsofpatientswithRAwithlessthanoneyearofsymptoms attheonsetoftreatmentwithDMARDs.12Thesecorrelations aresimilartothosefoundwiththeUS10scoringsystemin thepresentstudy.Theglobal12-jointUSscoringsystem vali-datedbyNaredoetal.(2008)identifiedmoderatecorrelations betweensynovialproliferationandbothPD(r=0.55)andthe DAS28(r=0.38).15 Perruconi et al.(2012)also found moder-atecorrelationsbetweenthenumberofjointswithsynovitis andboththeDAS28(r=0.53)andCRP(r=0.51)inasimplified six-jointscoringsystemadministeredtopatientswith estab-lishedRA.18ItshouldbestressedthattheUS10scoringsystem wasadministeredtoasmallnumberofjointsinpatientswith earlyRAandnoprioruse ofDMARDsand demonstrated a closerelationshipbetweentheUSfindingsanddisease activ-itycriteria,allowingbettermanagementofthesepatientsin clinicalpractice.
Thecorrelationsfoundinthepresentstudyarein disagree-mentwiththosereportedinpreviouscohortswithearlyRA. Naredoetal.(2007)foundmoderatecross-sectional correla-tionsbetweenjointcountanddiseaseactivityvariables(CRP andDAS28)atbaselineaswellasatsix,nineand12months offollowup,butfoundnolongitudinalcorrelationsbetween changesinUSevaluationandchangesintheclinicaland lab-oratoryfindings.
In the present study, a correlation was also found between the change in active synovitis (assessed based on PD parameters) and a changes in the HAQ score. This finding stronglysupports the hypothesis of an association betweenanimprovementinthesynovialinflammatory pro-cess and improvements in both function and quality of life.
FewstudieshaveinvestigatedinterobserverreliabilityinUS scoringsystems.Inthe12-jointsystemproposedbyNaredo etal.(2008),theauthorsfoundmoderateinterobserver agree-ment(k=0.5)regardingthepresenceofsynovialproliferation (whichissimilartotheagreementfoundinthepresentstudy) andastrongercorrelationthanthatreportedinthepresent studybetweensynovialproliferationandPD(r=0.8).15Inthe seven-jointsystemproposedbyBackhausetal.(2009), mod-erate agreement was found regarding the identification of synovialproliferation(k=0.62),withsomewhatlesser agree-ment (k=0.55)regardingthesemi-quantitativescore(0–3)16 and betteragreement(k=0.67) regardingthe qualitativePD variable,which issimilartothat ofthepresent study.The same study wasthe onlyprevious investigationtoaddress interobserverreliabilityinthedetectionofboneerosion,for whichmoderateagreementwasfound(k=0.56).16Regarding jointdamage,theUS10isthefirstglobal scoringsystemto addressthisevaluation,withexcellentagreementfoundfor the qualitativeand semi-quantitativevariables(k=0.79 and 0.82,respectively).
Thefindings ofthepresent study indicatethat the pro-posed US10 is a validscoring system for the follow up of inflammation and joint damagein patients withearly RA, demonstratingsignificantcorrelationswithclinicaland lab-oratoryfindingsaswellascorrelationsbetweenthechanges inthisscoreandbothclinicalandfunctionalmeasuresofthe diseasefollowingaspecifictreatmentprotocol.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.MangerB,KaldenJR.Jointandconnectivetissue ultrasonography–arheumatologicbedsideprocedure?A Germanexperience.ArthritisRheum.1995;38(6): 736–42.
2.GrassiW,CerviniC.Ultrasonographyinrheumatology:an evolvingtechnique.AnnRheumDis.1998;57(5):
268–71.
4. NewmanJS,AdlerRS,BudeRO,RubinJM.Detectionidsoft tissuehyperemia:valueofpowerDopplersonographyc.AmJ Roentgenol.1994;163(2):385–9.
5. BackhausM,KamradtT,SandrockD,LoreckD,FritzJ,WolfKJ, etal.Arthritisofthefingerjoints:acomprehensiveapproach comparingconventionalradiography,scintigraphy,
ultrasound,andcontrast-enhancedmagneticresonance imaging.ArthritisRheum.1999;42(6):1232–45.
6. WakefieldRJ,GibbonWW,ConaghanPG,O’ConnorPJ, McGonagleD,PeaseC,etal.Thevalueofsonographyinthe detectionofboneerosioninpatientswithrheumatoid arthritis.Acomparisonwithconventionalradiography. ArthritisRheum.2000;43(12):2762–70.
7. GrassiW,FilippucciE,FarinaA,CerviniC.Sonographic imagingoftendons.ArthritisRheum.2000;43(5): 969–76.
8. WakefieldRJ,O’ConnorPJ,ConaghanPG,McGonagleD, HensorEM,GibbonWW,etal.Fingertendondiseasein untreatedearlyrheumatoidarthritis:acomparisonof ultrasoundandmagneticresonanceimaging.Arthritis Rheum.2007;57(7):1158–64.
9. ScheelAK,HermannKA,KahlerE,PasewaldtD,FritzJ,Hamm B,etal.Anovelultrasonographicsynovitisscorringsystem suitableforsnalysingfingerjointinflammationin rheumatoidarthritis.ArthritisRheum.2005;52(3): 733–43.
10.SzkudlarekM,Court-PayenM,JacobsenS,KlarlundM, ThomsenH,OstergaardM.Interobserveragreementin ultrasonographyofthefingerandtoejointsinrheumatoid arthritis.ArthritisRheum.2003;48(4):955–62.
11.FilippucciE,FarinaA,CarottiM,SalaffiF,GrassiW.Greyscale andpowerDopllersonographicchangesinducesby
intra-articularsteroidinjectiontreatment.AnnRheumDis. 2004;63(6):740–3.
12.NaredoE,BonillaG,GameroF,UsonJ,CarmonaL,LaffonA. Assessmentofinflammatoryactivityinrheumatoidarthritis: acomparativestudyofclinicalevaluationwithgreyscaleand powerDopplerultrasonography.AnnRheumDis.
2005;64(3):375–81.
13.BrownAK,QuinnMA,KarimZ,ConaghanPG,PeterfyCG, HensorE,etal.Presenceofsignificantsynovitisin rheumatoidarthritispatientswithdisease-modifying antirheumaticdrug-inducedclinicalremission:evidence fromanimagingstudymayexplainstructuralprogression. ArthritisRheum.2006;54(12):3761–73.
14.LuzKR,FurtadoR,MitraudSV,PorglhofJ,NunesC,Fernandes AR,etal.Interobserverreliabilityinultrasoundassessmentof rheumatoidwristjoints.ActaReumatolPort.
2011;36(3):245–50.
15.NaredoE,RodríguezM,CamposC,Rodríguez-HerediaJM, MedinaJA,GinerE,etal.Validity,reproducibility,and responsivenessofatwelve-jointsimplifiedpowerdoppler ultrasonographicassessmentofjointinflammationin rheumatoidarthritis.ArthritisRheum.2008;59(4): 515–22.
16.BackhausM,OhrndorfS,KellnerH,StrunkJ,BackhausTM, HartungW,etal.Evaluationofanovel7-jointultrasound scoreindailyrheumatologicpractice:apilotproject.Arthritis Rheum.2009;61(9):1194–201.
17.DougadosM,Jousse-JoulinS,MistrettaF,d’AgostinoMA, BackhausM,BentinJ,etal.Evaluationofseveral ultrasonographyscoringsystemsforsynovitisand comparisontoclinicalexamination:resultsfroma prospectivemulticentrestudyofrheumatoidarthritis.Ann RheumDis.2010;69(5):828–33.
18.PerriconeC,CeccarelliF,ModestiM,VavalaC,DiFrancoM, ValesiniG,etal.The6-jointultrasonographicassessment:a valid,sensitive-to-changeandfeasiblemethodforevaluating
jointinflammationinRA.Rheumatology(Oxford). 2012;51(5):866–73.
19.Jacoby,JaysonRK,CoshMIV,OnsetJA.Earlystagesand prognosisofrheumatoidarthritis:aclinicalstudyof100 patientswith11yearfollow-up.BrMedJ.1973;2(5858): 96.
20.HamalainenM,KammonenM,LethimakiM.Epidemiologyof wristinvolvementinrheumatoidartritis.Rheumatol. 1992;17:1–7.
21.VisserH,leCessieS,VosK,BreedveldFC,HazesJM.Howto diagnoserheumatoidarthritisearly:apredictionmodelfor persistent(erosive)arthritis.ArthritisRheum.
2002;46(2):357–65.
22.TanAL,TannerSF,ConaghanPG,RadjenovicA,O’ConnorP, BrownAK,etal.Roleofmetacarpophalangealjointanatomic factorsinthedistributionofsynovitisandboneerosionin earlyrheumatoidarthritis.ArthritisRheum.
2003;48(5):1214–22.
23.ArnettFC,EdworthySM,BlochDA,McShaneDJ,FriesJF, CooperNS,etal.TheAmericanRheumatismAssociation 1987revisedcriteriafortheclassificationofrheumatoid arthritis.ArthritisRheum.1988;31(3):315–24.
24.AletahaD,NeogiT,SilmanAJ,FunovitsJ,FelsonDT,Bingham CO,etal.2010RheumatoidArthritisClassificationCriteriaAn AmericanCollegeofRheumatology/EuropeanLeagueAgainst RheumatismCollaborativeInitiative.ArthritisRheum. 2010;62(9):2569–81.
25.vanderHelm-vanMilAH,leCessieS,vanDongenH, BreedveldFC,ToesRE,HuizingaTW.Apredictionrulefor diseaseoutcomeinpatientswithrecent-onset
undifferentiatedarthritis:howtoguideindividualtreatment decisions.ArthritisRheum.2007;56(2):433–40.
26.FerrazMB,OliveiraLM,AraújoPM,AtraE,TugwellP. Crossculturalreliabilityofthephysicalabilitydimensionof thehealthassessmentquestionnaire.JRheumatol. 1990;17(6):813–7.
27.BackhausM,BurmesterGR,GerberT,GrassiW,MacholdKP, SwenWA,etal.Guidelinesformusculoskeletalultrasoundin rhematology.AnnRheumDis.2001;60(7):641–9.
28.WakefieldRJ,BalintPV,SzkudlarekM,FilippucciE,Backhaus M,D’AgostinoMA,etal.Musculoskeletalultrasound includingdefinitionsforultrasonographicpathology.J Rheumatol.2005;32(12):2485–7.
29.GrassiW,TittarelliE,PiraniO,AvaltroniD,CerviniC. Ultrasoundexaminationofmetacarpophalangealjointsin rheumatoidarthritis.ScandJRheumatol.1993;22(5): 243–7.
30.BoutryN,LardéA,DemondionX,CortetB,CottenH,Cotton A.MetacarpophalangealjointsatUSinasymptomatic volunteersandcadavericspecimens.Radiology. 2004;232(3):716–24.
31.MöllerB,BonelH,RotzetterM,VilligerPM,ZiswilerHR. Measuringfingerjointcartilagebyultrasoundasapromising alternativetoconventionalradiographimaging.Arthritis Rheum.2009;61(4):435–41.
32.FilippucciE,daLuzKR,DiGesoL,SalaffiF,TardellaM,Carotti M,etal.Interobserverreliabilityofultrasonographyinthe assessmentofcartilagedamageinrheumatoidarthritis.Ann RheumDis.2010;69(10):1845–8.
33.vanderHeijdeDM.PlainX-raysinrheumatoidarthritis: overviewofscoringmethods,theirreliabilityand applicability.BaillieresClinRheumatol.1996;10(3): 435–53.
34.LandisJR,KochGG.Themeasurementofobserveragreement forcategoricaldata.Biometrics.1977;33(1):159–74.
36.NaredoE,ColladoP,CruzA,PalopMJ,CaberoF,RichiP,etal. LongitudinalpowerDopplerultrasonographicassessmentof jointinflamatoryactivityinearlyrheumatoidarthritis: predictivevalueindiseaseactivityandradiologic progression.ArthritisRheum.2007;57(1):116–24.
37.ScirèCA,MontecuccoC,CodulloV,EpisO,TodoertiM, CaporaliR.Ultrasonographicevaluationofjointinvolvement
inearlyrheumatoidarthritisinclinicalremission:power Dopplersignalpredictsshort-termrelapse.Rheumatology (Oxford).2009;48(9):1092–7.
38.HammerHB,KvienTK.Ultrasonographyshowssignificant improvementinwristandankletenosynovitisinrheumatoid arthritispatientstreatedwithadalimumab.ScandJ