w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
Hyperuricemia
in
systemic
lupus
erythematosus:
is
it
associated
with
the
neuropsychiatric
manifestations
of
the
disease?
Mahdi
Sheikh
a,
Shafieh
Movassaghi
a,∗,
Mohammad
Khaledi
b,
Maryam
Moghaddassi
aaTehranUniversityofMedicalSciences,RheumatologyResearchCenter,Tehran,Iran
bTehranUniversityofMedicalSciences,Imam-KhodeminiHospital,DepartmentofNeurology,Tehran,Iran
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received25February2015
Accepted12May2015
Availableonline10August2015
Keywords:
Neurology Neuropathy
Systematiclupuserythematosus
Stroke
Uricacid
a
b
s
t
r
a
c
t
Objectives: Toassesstheassociationbetweenhyperuricemiaanddifferentneuropsychiatric
manifestationsandstrokeriskfactorsinsystematiclupuserythematosus(SLE)patients.
Methods:Thisstudywasconductedon204SLEpatientswhowereadmittedtoatertiary
referralcenter.Astandardizedquestionnairewascompletedforalltheparticipantsand
themedicalrecordswerereviewedregardingtheoccurrenceofarterialorvenous
throm-boticevents,stroke,seizure,depression,headache,psychosis,andperipheralneuropathy.
Inadditionbloodsamplesweredrawntoobtainserumuricacid,triglyceride(TG),
high-densitylipoprotein(HDL)cholesterol,low-densitylipoprotein(LDL)cholesterol,andtotal
cholesterollevels.
Results:Hyperuricemia(serumuricacid≥6mg/dlforwomenand≥7mg/dlformen)was
detectedin16.1%ofSLEpatientsandwassignificantlyassociatedwiththeoccurrenceof
stroke(OR,2.38;95%CI,1.2–7.24),andperipheralneuropathy(OR,3.49;95%CI,1.52–12.23),
independent ofhypertensionand hyperlipidemia.Hyperuricemiawasalso significantly
associatedwithhypertension(OR,7.76;95%CI,2.72–15.76),hyperlipidemia(OR,5.05;95%
CI,1.59–11.32),andhistoryofarterialthrombosis(OR,4.95;95%CI,1.98–15.34),independent
ofageandbodymassindex.
Conclusions: HyperuricemiainSLEpatientsisindependentlyassociatedwiththeoccurrence
ofstrokeandperipheralneuropathy.Itisalsoindependentlyassociatedwith
hyperten-sion,hyperlipidemia,andhistoryofarterialthrombosis,whicharethemajorstrokeand
myocardialinfarctionriskfactorsinSLEpatients.
©2015ElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-ND
license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthor.
E-mail:dr.s.movassaghi@gmail.com(S.Movassaghi).
http://dx.doi.org/10.1016/j.rbre.2015.07.011
2255-5021/©2015ElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/
Hiperuricemia
no
lúpus
eritematoso
sistêmico:
está
associada
a
manifestac¸ões
neuropsiquiátricas
da
doenc¸a?
Palavras-chave:
Neurologia Neuropatia
Lúpuseritematososistêmico
Acidentevascularencefálico
Ácidoúrico
r
e
s
u
m
o
Objetivos: Avaliaraassociac¸ãoentreahiperuricemiaediferentesmanifestac¸ões
neurop-siquiátricaseosfatoresderiscoparaAVEempacientescomlúpuseritematososistêmico
(LES).
Métodos: Esteestudofoirealizadoem204pacientescomLESqueforaminternadosem
um centro de referênciadeatenc¸ão terciária.Todos os participantespreencheram um
questionáriopadronizadoeosprontuáriosmédicosforamanalisadosquantoà
ocorrên-ciadeeventostrombóticosarteriaisouvenosos,acidentevascularencefálico,convulsão,
depressão,cefaleia,psicoseeneuropatiaperiférica.Alémdisso,foramcoletadasamostras
desangueparasemensurarosníveisdeácidoúrico,triglicerídeos(TG),lipoproteínasde
altadensidade(HDL),lipoproteínasdebaixadensidade(LDL)ecolesteroltotaldosangue.
Resultados: Ahiperuricemia(ácidoúricosérico≥6mg/dlparamulherese≥7mg/dlpara
homens)foidetectadaem16,1%dospacientescomLESeestevesignificativamente
asso-ciadaàocorrênciadeAVE(OR,2,38;IC95%,1,2–7,24)eneuropatiaperiférica(OR,3,49;IC
95%,1,52–12,23),independentementedahipertensãoarterialedahiperlipidemia.A
hipe-ruricemiatambémestevesignificativamenteassociadaàhipertensãoarterial(OR,7,76;IC
95%,2,72–15,76),hiperlipidemia(OR,5,05;IC95%,1,59–11,32)ehistóriadetrombosearterial
(OR,4,95;95%CI,1,98–15,34),independentementedaidadeeíndicedemassacorporal.
Conclusões: AhiperuricemiaempacientescomLESestáindependentementeassociadaà
ocorrênciadeacidentevascularencefálicoeneuropatiaperiférica.Tambémestá
indepen-dentementeassociadaàhipertensão,hiperlipidemiaehistóriadetrombosearterial,quesão
osprincipaisfatoresderiscoparaacidentevascularencefálicoeinfartoagudodomiocárdio
empacientescomLES.
©2015ElsevierEditoraLtda.Este ´eumartigoOpenAccesssobumalicenc¸aCC
BY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Uricacid,thefinalproductofpurinedegradation,isformedin
theliverfromprecursorproteinsandisexcretedbythe
kid-neysandintestines.Atphysiologicconcentrations,uricacid
exhibits excellent antioxidant activity;however, when uric
acidexceedsitsphysiologiclevels,itcanpropagateoxidative
damage. Furthermore, chronic elevation of uric acid
con-stitutes arisk factor formany diseases,as it can promote
inflammationandendothelialdysfunction.1,2
Hyperuricemia is arisk factor for myocardial infarction
andstroke.3Inadditionhigherserumuratelevelsafteracute
stroke is a predictor of pooroutcome and higher rates of
future vascular event.4 Hyperuricemia has been also
asso-ciated with peripheral neuropathy in diabetes.5 Therefore
someresearchersrecommended loweringplasma uricacid
levelstoreducetheriskoffuturevasculareventsinhighrisk
populations.4,6
Neurologicinvolvementandvasculareventshaveawide
rangeoffrequency(12–95%)inpatientswithsystemiclupus
erythematosus(SLE)andcanbeverycommoncausing
signif-icantmorbidityandmortalityinSLEpatients.7Basedonthe
studiesthatshowedhigherlevelsofuricacidinSLEpatients,8,9
andthestudiesthatdocumentedtheinjuriouseffectofuric
acidonthenervoussystem,6wepostulatethathyperuricemia
inSLEpatientsmightincreasetheriskofneurologic
involve-mentandvasculareventsduringthecourseofthedisease.By
ourextensivesearchwecouldnotfindstudiesassessingthe
relationbetweenserumuricacidlevelsandthedifferent
neu-ropsychiatricmanifestationsofSLE.Whetherahigh serum
uricacidlevelinSLEpatientsconstitutesariskfactorforfuture
neurologic, psychiatricandvascular involvements,remains
unknown.Identifyingtheseassociationsisveryimportantand
mighthelpinidentifyingamodifiableriskfactorforthe
neu-rologicandvasculareventsinSLEpatients.
Weundertookthis studytoevaluatetheeffectof
hyper-uricemiaonthedifferentneurologicmanifestationsseenin
SLEpatients;wealsoattemptedassessingtheassociationsof
serumuricacidlevelswiththepatients’bloodpressureand
lipidprofile,andtheoccurrenceofvascularandthrombotic
events.
Materials
and
methods
Studypopulationandstudydesign
ThisstudywasconductedonSLEpatientswhowere
admit-tedatourcenter(atertiaryreferralhospital)betweenMarch
2011andFebruary2014.Atotalof235SLEpatientswhomet
theAmericanCollegeofRheumatology(ACR)SLEcriteria
par-ticipatedinthestudy,10 and31ofthesewereexcludeddue
tothefollowingcriteria:historyofsmoking;opiateor
alco-holconsumption;andhistoryofinfections,fever,orantibiotic
useduringtheprevioustwoweeks.Afterobtainingawritten
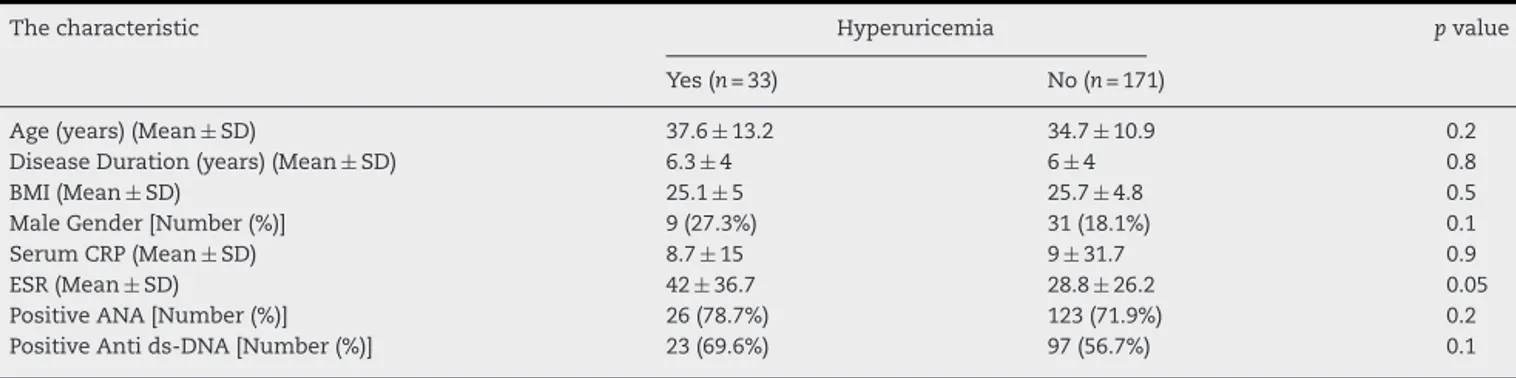
Table1–Demographiccharacteristicsandsomelaboratorymarkersoftheparticipantswithandwithouthyperuricemia.
Thecharacteristic Hyperuricemia pvalue
Yes(n=33) No(n=171)
Age(years)(Mean±SD) 37.6±13.2 34.7±10.9 0.2
DiseaseDuration(years)(Mean±SD) 6.3±4 6±4 0.8
BMI(Mean±SD) 25.1±5 25.7±4.8 0.5
MaleGender[Number(%)] 9(27.3%) 31(18.1%) 0.1
SerumCRP(Mean±SD) 8.7±15 9±31.7 0.9
ESR(Mean±SD) 42±36.7 28.8±26.2 0.05
PositiveANA[Number(%)] 26(78.7%) 123(71.9%) 0.2
PositiveAntids-DNA[Number(%)] 23(69.6%) 97(56.7%) 0.1
SD,standarddeviation;CRP,c-reactiveprotein;ESR,erythrocytesedimentationrate;ANA,antinuclearantibody;Antids-DNA,antidouble strandDNA.
completedthestudy,whichwasapprovedbytheethics com-mitteeandtheresearchdeputyofourinstitute.
Dataandspecimencollection
Upon enrollment a standardized questionnaire was
com-pleted for every participant through interviews, medical
records, and physical examinations. The questionnaire
consistedofdemographic, medical,and socialhistories, as wellasinquiriesaboutbodymassindex(BMI),disease dura-tion,andthereceivedtreatmentsandtheirduration.Patients fileswere investigatedand thefollowing informationwere
recorded: the occurrence of arterial or venous thrombotic
eventsdocumentedbyimagingstudies,occurrenceofstroke documentedbyimagingstudies,recentonsetseizure
demon-strated by an abnormal electroencephalography (EEG) that
wasnotduetoinfectionormetabolicdisturbances,the
pres-ence of depression, headache, psychosis due to lupus as
definedbytheACRandSLEdiseaseactivityindex(SLEDAI),10,11
andperipheralneuropathydocumentedbyelectromyography
(EMG)andnerveconductionvelocity(NCV)studies.Inaddition
uponenrollmentbloodsamplesweredrawntoobtainserum
uricacid,creatinine,bloodurea nitrogen(BUN),triglyceride
(TG),high-densitylipoprotein(HDL)cholesterol,low-density
lipoprotein(LDL)cholesterol,andtotalcholesterollevels.Uric
acid levelswere determined bythe enzymatic colorimetric
method,andalllaboratoryinvestigationswereperformedby
onepersonwhowasblindedtotheresultsofthe
question-naires.
Statisticalanalysis
AllstatisticalanalyseswereperformedusingSPSSstatistical
software(version18.0.0: PASW, Chicago,IL).TheChi-square
analysis,Fisher’sexacttest,independentsamplest-test,
one-wayanalysis of variance, and Pearson correlation analysis
were used to analyze the correlations and relationships
between the variables. Multivariate logistic regression was
usedtoevaluatethedependencyoftheobtainedresults.
Sam-plesizewascalculatedforanalphaerrorof0.05,adesired
levelofabsoluteprecision(d)of0.05,andanestimateddesign
effect(DEFF)ofone.Estimatedoddsratios(OR)with95%
confi-denceintervals(95%CI)andpvalueswereusedtoevaluatethe
statistical significance of the associations and correlations
betweenthevariables.
Results
Descriptivestatistics
Atotalof235SLEpatientsagreedtoparticipateinthestudy;
ofthese,31patientswereexcluded.Sixteenofthe31excluded
patientshadahistoryofsmokingandopiateoralcohol
con-sumption,and15hadinfectionsorhadusedantibioticsduring
the previous two weeks. The remaining 204 SLE patients
completedthestudy.Atenrollment,thepopulation
charac-teristicsexpressedasmean±standarddeviation(SD)wereas
follows:participants’age,35.3±11.4years;diseaseduration,
6±4 years; BMI, 25.6±4; serum creatinine, 0.9±0.4mg/dl;
BUN,20.4±9.2mg/dl;andserumuricacid,4.7±1.5mg/dl.
No significant differences were observed in the
demo-graphics of the SLE patients with and without
hyper-uricemia (Table 1). There were no significant differences
between the drugs usedby the participantsin each group
(Table2).
Oftheparticipantswho completedthe study,40(19.6%)
were male, 69 (33.8%) had hypertension (defined as
sys-tolic bloodpressure ≥140mmHgor diastolicbloodpressure
≥90mmHg or being treated with hypertensive drugs), 43
(21%)hadhyperlipidemia(definedasserumtotalcholesterol
≥240mg/dl,LDLcholesterol≥160mg/dl,orTG≥200mg/dl),12
Table2–Thedrugsusedbytheparticipantswith andwithouthyperuricemia.
Thedrug Hyperuricemia pvalue
Yes(n=33) No(n=171)
Prednisolone 33(100%) 166(97%) 0.4
Hydroxychloroquine 16(48.4%) 102(59.6%) 0.1
Cellcept 13(39.3%) 57(33.3%) 0.3
Azathioprine 7(21.2%) 48(28%) 0.2
Aspirin 3(9%) 20(11.6%) 0.4
Statins 4(12.1%) 26(15.2%) 0.4
Table3–AssociationbetweenhyperuricemiaanddifferentneuropsychiatricmanifestationsofSLE.
NeurologicmanifestationsofSLE Hyperuricemia UnadjustedOR
(95%CI)
AdjustedORa (95%CI)
Yes(n=33) No(n=171)
N(%) N(%)
CVA(n=19) 8(24.2%) 11(6.4%)b 4.65(1.7–12.69) 2.38(1.2–7.24)
Seizure(n=22) 5(15.1%) 17(9.9%) 1.61(0.55–4.74) 1.4(0.45–7.7)
Headache(n=55) 16(48.5%) 39(22.8%)c 3.18(1.47–6.88) 2.74(0.98–9.88)
Peripheralneuropathy(n=12) 6(18.2%) 6(3.5%)b 6.11(1.83–20.34) 3.49(1.52–12.23)
Psychosis(n=10) 4(12.1%) 6(3.5%)c 3.79(1–14.27) 3.32(0.9–16.11)
Depression(n=23) 5(15.2%) 18(10.5%) 1.51(0.52–4.42) 1.1(0.35–5.64)
SLE,systemiclupuserythematosus;OR,oddsratio;95%CI,95%confidenceinterval;CVA,cerebrovascularaccident.
a Adjustedforhypertensionandhyperlipidemia.
b p<0.05forthecomparisonbetweentwogroupswithandwithouthyperuricemiaafterperforminglogisticregression.
c p<0.05forthecomparisonbetweentwogroupswithandwithouthyperuricemia.
22(10.7%)hadexperiencedatleastoneseizureattack,10(4.9%)
hadpsychosis,23(11.27%)haddepression,55(26.9%)hada
severeheadache,19(9.3%)hadexperiencedCVA,12(5.8%)had
peripheralneuropathy,50(24.5%)hadexperiencedatleastone
venousthrombosisformation,and20(9.8%)hadexperienced
atleastonearterialthrombusformation.
Theassociationbetweenhyperuricemiaandthedifferent neurologicmanifestationsofSLE
Hyperuricemiawasdefinedasserumuricacid≥6mg/dlfor
women and serum uric acid ≥7mg/dl for men.13
Hyper-uricemia was detectedin 33 SLEpatients (16.1%) and was
significantlyassociatedwiththeoccurrenceofCVA(p=0.001),
psychosis (p=0.03), peripheral neuropathy (p=0.001), and
headache (p=0.003). There was no statistically significant
associationbetween hyperuricemiaandseizures (p=0.3)or
depression(p=0.4)(Table3).
Theassociationsbetweenserumuricacidlevelsandthe differentknownCVAriskfactors
Based on the Pearson correlation coefficients, serum uric
acidlevelsweresignificantlycorrelatedwithbloodpressure
(r=0.5,p=0.000),totalcholesterol(r=0.3,p=0.000),TG(r=0.03,
p=0.000),andLDLcholesterol(r=0.2,p=0.004).Wedidnotfind
astatisticallysignificantcorrelationbetweenserumuricacid
levelsandtheparticipants’age,BMI,orHDLcholesterollevel.
Hyperuricemiawasassociatedwithhypertension(p=0.000),
hyperlipidemia(p=0.000),andarterialthrombosis(p=0.000),
while there was no significant association between
hyper-uricemiaandvenousthrombosis(p=0.3)(Table4).
Dependencyoftheobtainedresults
Using multivariate logistic regression,after adjustmentfor
hypertension and hyperlipidemia, hyperuricemia remained
significantly associated with CVA (B=0.87, p=0.04) and
peripheral neuropathy (B=1.25, p=0.04) but not with
psychosis (B=1.2, p=0.1) or headache (B=1.01, p=0.05)
(Table3).
AfteradjustmentforageandBMI,hyperuricemiaremained
significantlyassociatedwithhypertension(B=2.05,p=0.000),
hyperlipidemia (B=1.62, p=0.006), and arterial thrombosis
(B=1.6,p=0.001)(Table4).
Discussion
HyperuricemiaandSLE
Inthecurrentstudy,hyperuricemiawasdetectedin16.1%of
SLEpatients(22%inmenand16.4%inwomen),whichwas
higherthan theprevalenceofhyperuricemiainthenormal
Table4–AssociationbetweenhyperuricemiaanddifferentknownriskfactorsofCVA.
Factors Hyperuricemia UnadjustedOR
(95%CI)
AdjustedORa (95%CI)
Yes(n=33) No(n=171)
N(%) N(%)
Hypertension(n=69) 25(75.8%) 44(25.7%)b 9.02(3.79–21.46) 7.76(2.72–15.76)
Hyperlipidemia(n=43) 17(51.5%) 26(15.2%)b 5.92(2.66–13.19) 5.05(1.59–11.32)
Hxofarterialthrombosis(n=20) 9(27.3%) 11(6.4%)b 5.45(2.04–14.53) 4.95(1.98–15.34)
Hxofvenousthrombosis(n=50) 10(30.3%) 40(23.4%) 1.42(0.62–3.24) 1.22(0.54–5.67)
OR,oddsratio;95%CI,95%confidenceinterval;CVA,cerebrovascularaccident;Hx,history.
a Adjustedforageandbodymassindex(BMI).
population, as reported by studies conducted in the same
regionusingthesamecutoffpoints.14 Thisisinaccordance
with other studies that showed higher levels of uric acid
amongSLEpatients.8,9Higherprevalenceofhyperuricemiain
SLEpatientsmightbeduetoseveralendogenousand
exoge-nousmechanismssuchasinflammation,hypertension,and
renalinvolvement,whichare prevalentinSLEpatientsand
havebeenidentifiedasprovokinghyperuricemiathrough
dif-ferentmechanisms.15–19 Ontheotherhand,increasedlevels
ofuricacid canaggravateinflammation,hypertension,and
renaldisease,15–19thuscreatingaviciouscycle.Hyperactivity
ofthexanthineoxidaseenzymeinSLEpatients,8andsomeof
thedrugsusedinthetreatmentofSLE,20areamongtheother
possiblereasonsforthehigherprevalenceofhyperuricemia
inSLEpatients.
Hyperuricemiaandhyperlipidemia
Inourstudy,theserumuricacidlevelwassignificantly
cor-relatedwiththeserumTG,LDL,andtotalcholesterollevels,
andhyperuricemiawassignificantlyassociatedwith
hyper-lipidemia,independentofageandBMI.Thesefindingsarein
accordancewithotherstudiesthatinvolvedbothhumanand
animalmodels.21,22Hyperuricemiaappearstohaveamutual
interactionwithhighserumTGandcholesterollevels,thus
formingaviciouscycle, whereassomestudieshaveshown
thatlipidsandhypertriglyceridemiaincreaseserumuricacid
levelsthroughincreasingitsabsorptionintherenaltubules
and alsothrough increasinguricacid production by
accel-erating thede novopurinesynthesis.23 Other studies have
documentedthaturicacidmighthaveacontributoryrolein
increasingserumTG,LDL,andtotalcholesterollevels.
Naka-gawaetal.,intheirstudyoftheeffectofuricacidonmetabolic
syndrome, documented that lowering uric acid improves
insulin sensitivity, obesity, and hypertriglyceridemia. They
alsoindicatedthaturicacidmightbeinvolvedineitherthe
overproductionorthereductionofTGclearance.21Inanother
study,Bowdenetal.documentedthathyperuricemiais
asso-ciatedwithhighertotalcholesterol,LDL,andapolipoproteinB
(ApoB)levels, andareductioncauses adecreaseinserum
LDL and total cholesterol levels. They indicated that uric
acidisamajorcauseofoxidativestressandreducednitrous
oxide (NO) release,and combined with anincrease in the
activity of lipoproteinlipase may cause higher lipid levels
andparticlenumbers.Furthermore,hyperuricemiaisthought
to impair endothelium-dependent vasodilatation primarily
throughlipidoxidation,whichcancauseanincreaseinthe
totalcholesterollevel.22
Hyperuricemiaandhypertension
In this study of SLE patients, hyperuricemia was
signifi-cantlyassociatedwithhypertension,independentofageand
BMI.Graysonet al.intheirmeta-analysisofhyperuricemia
and incident hypertension, which included data from
55,607 patients, found a significantly increased adjusted
risk ratio for incident hypertension in subjects with
hyperuricemia, independent of traditional risk factors for
hypertension.15Itisnowbelievedthathyperuricemiahasa
causativeroleinhypertensionthroughdifferentmechanisms;
uricacidactivatestherenin–angiotensinsystemand
down-regulates nitric oxide (NO) production, thus leading to
vasoconstriction. Another effect of uric acid, which
devel-ops overtime, is uric acid mediated arteriolosclerosis; uric
acid uptakeinto vascular smooth muscle cells causes the
activation and production ofgrowth factors and monocyte
chemoattractantprotein-1,whichresultsinvascularsmooth
musclecellproliferation,vascularwallthickening,lossof
vas-cularcompliance,andashiftinpressurenatriuresis.1,2,15–17
Hyperuricemia,hypercoagulabilitystate,andCVA
Inthecurrentstudy,hyperuricemiawassignificantly
associ-atedwithCVAinSLEpatients,independentofhypertension
and hyperlipidemia.Thisisinaccordance withother
stud-ies conductedingeneralpopulations. Arecentlypublished
12–15yearsprospectivestudybyStorhaugetal.thatincluded
5700participantswithoutknownriskfactors for
cardiovas-culardiseasesdocumentedthata1SD(1.47mg/dl)increase
inserum uric acidwas significantlyassociatedwitha 22%
increased risk for ischemic stroke and 13% increased risk
for all-cause mortality.24 Additionally, in our study,
hyper-uricemiawasindependentlyassociatedwithahistoryofat
leastonearterialthrombosisevent.Theseimportantfindings
suggestthathyperuricemiamightincreasetheriskforCVA
notonlybyincreasingtheriskfordevelopinghyperlipidemia
andhypertensionasmentionedearlier,butalsothroughother
mechanisms;hyperuricemiahasbeenassociatedwithplatelet
activation and increased platelet adhesiveness.25,26 Thus,
patients with hyperuricemia might have anincreased risk
ofthrombusformation.Inaddition,hyperuricemiahasbeen
associatedwiththeprogressionofatherosclerosisthroughthe
promotionofoxygenationofLDLcholesterolandfacilitationof
lipidperoxidation.3,4,6Furthermore,hyperuricemiacancause
endothelialdysfunctionandreduceNOproductionleadingto
animpairedvasculartonethatcouldcontributetoischemic
changes.1,2,15,24
Hyperuricemiaandperipheralneuropathy
Inourstudy,hyperuricemiawassignificantlyassociatedwith
peripheralneuropathyinSLEpatients,independentof
hyper-tensionandhyperlipidemia.Thisimportantfindingsuggests
that hyperuricemia might have an injurious effect on the
peripheralnervoussystem.Similarresultswerefoundin
dia-betic patients. Papanaset al., in their study of64 diabetic
patients, detected a significant correlation between serum
uric acid and the neuropathy disability score. They also
indicatedthatdiabetic patientswithperipheralneuropathy
had increasedserumuricacid levelscomparedtolevelsin
those without neuropathy.5 The exact role of uric acid in
peripheralneuropathyremainsunknown;however,uricacid
mightplayaroleinperipheralneuropathythroughitsrole
in oxidative damageand vascular endothelial dysfunction.
Studieshaveshownthatwhenuricacidexceedsits
physio-logicvalueintheplasma,itcanpropagateoxidativedamage
and cause oxidative stress,1,2,22 which has been shown to
induce neuronal damage; oxidative stress is the central
mediatorofapoptosis,neuro-inflammation,andbioenergetic
inflammationandendothelialdysfunction,andreducesNO
production and bioavailability.1,2,15 Endothelial dysfunction
and low levels of NO may lead to constriction of blood
vessels supplying the nerve, which contributes to nerve
damage.28
Conclusion
Hyperuricemia isprevalent amongSLEpatientsand is
sig-nificantlyassociatedwithCVAandperipheralneuropathyin
SLEpatients. It isalso significantly associatedwith
hyper-tension, hyperlipidemia, and a positive history for arterial
thrombosis,whicharethemajorCVAandmyocardial
infarc-tion riskfactors.Follow up studies are neededto measure
serum uric acid atthe beginning and through the course
of the disease to evaluate the effect of hyperuricemia on
the progression of neuropsychiatric manifestations of SLE
and itsmorbidity throughthe courseofthe disease.
Addi-tionallyinterventionalstudiesare neededtodeterminethe
practicalusefulnessofloweringserumuricacidlevelsinSLE
patients.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorsthankDr.SepidehSeifiandDr.AmirMirbagheri
fortheircontributionindesigningthestudy.Thisresearchwas
fundedbytheRheumatologyResearchCenter.
r
e
f
e
r
e
n
c
e
s
1. DeOliveiraEP,BuriniRC.Highplasmauricacidconcentration: causesandconsequences.DiabetolMetabSyndr.2012;4:12.
2. SautinYY,JohnsonRJ.Uricacid:theoxidant–antioxidant paradox.NucleosidesNucleotidesNucleicAcids. 2008;27:608–19.
3. BosMJ,KoudstaalPJ,HofmanA,WittemanJC,BretelerMM. Uricacidisariskfactorformyocardialinfarctionandstroke: theRotterdamstudy.Stroke:JCerebCircul.2006;37:1503–7.
4. WeirCJ,MuirSW,WaltersMR,LeesKR.Serumurateasan independentpredictorofpooroutcomeandfuturevascular eventsafteracutestroke.Stroke:JCerebCircul.
2003;34:1951–6.
5. PapanasN,KatsikiN,PapatheodorouK,DemetriouM, PapazoglouD,GiokaT,etal.Peripheralneuropathyis associatedwithincreasedserumlevelsofuricacidintype2 diabetesmellitus.Angiology.2011;62:291–5.
6. KanellisJ,JohnsonRJ.Editorialcomment–Elevateduricacid andischemicstroke:accumulatingevidencethatitis injuriousandnotneuroprotective.Stroke:JCerebCircul. 2003;34:1956–7.
7. KampylafkaEI,AlexopoulosH,KosmidisML,Panagiotakos DB,VlachoyiannopoulosPG,DalakasMC,etal.Incidenceand prevalenceofmajorcentralnervoussysteminvolvementin systemiclupuserythematosus:a3-yearprospectivestudyof 370patients.PLOSONE.2013;8:e55843.
8.NikolenkoIuI,SiniachenkoOV,Anan’evaMN,NikolenkoV, DubiagaVV,ShchukinIN.Hyperuricemiaanddisordersin contentofaminoacids-purineprecursorsinpatientswith autoimmunediseasesandgout.Likars’kasprava/Ministerstvo okhoronyzdorov’iaUkrainy;2005.p.34–6.
9.SantosMJ,VinagreF,SilvaJJ,GilV,FonsecaJE.Cardiovascular riskprofileinsystemiclupuserythematosusandrheumatoid arthritis:acomparativestudyoffemalepatients.Acta ReumatolPort.2010;35:325–32.
10.HochbergMC.UpdatingtheAmericanCollegeof Rheumatologyrevisedcriteriafortheclassificationof systemiclupuserythematosus.ArthritisRheum. 1997;40:1725.
11.BombardierC,GladmanDD,UrowitzMB,CaronD,ChangCH. DerivationoftheSLEDAIAdiseaseactivityindexforlupus patients.TheCommitteeonPrognosisStudiesinSLE. ArthritisRheum.1992;35:630–40.
12.ThirdreportoftheNationalCholesterolEducationProgram (NCEP)ExpertPanelondetection,evaluation,andtreatment ofhighbloodcholesterolinadults(AdultTreatmentPanelIII) finalreport.Circulation.2002;106:3143–421.
13.ConenD,WietlisbachV,BovetP,ShamlayeC,RiesenW, PaccaudF,etal.Prevalenceofhyperuricemiaandrelationof serumuricacidwithcardiovascularriskfactorsina developingcountry.BMCPublicHealth.2004;4:9.
14.SadrSM,NamayandehSM,MoadaresMM,RafieiM.Serum uricacidlevelsanditsassociationwithcardiovascularrisk factors.IranJPublicHealth.2009;38:53–9.
15.GraysonPC,KimSY,LaValleyM,ChoiHK.Hyperuricemiaand incidenthypertension:asystematicreviewand
meta-analysis.ArthritisCareRes.2011;63:102–10.
16.FeigDI.Hyperuricemiaandhypertension.1.Advancesin chronickidneydisease.2012;19:377–85.
17.KrishnanE.Interactionofinflammation,hyperuricemia,and theprevalenceofhypertensionamongadultsfreeof metabolicsyndrome:NHANES2009–2010.JAmHeartAssoc. 2014;3:e000157.
18.YangZ,LiangY,XiW,ZhuY,LiC,ZhongR.Associationof serumuricacidwithlupusnephritisinsystemiclupus erythematosus.RheumatolInt.2011;31:743–8.
19.ZhouY,FangL,JiangL,WenP,CaoH,HeW,etal.Uricacid inducesrenalinflammationviaactivatingtubularNF-kappaB signalingpathway.PLOSONE.2012;7:e39738.
20.MazzaliM.Uricacidandtransplantation.SeminNephrol. 2005;25:50–5.
21.NakagawaT,HuH,ZharikovS,TuttleKR,ShortRA, GlushakovaO,etal.Acausalroleforuricacidin
fructose-inducedmetabolicsyndrome.AmJRenalPhysiol. 2006;290:F625–31.
22.BowdenRG,ShelmadineBD,MoreillonJJ,DeikeE,GriggsJO, WilsonRL.EffectsofuricacidonlipidlevelsinCKD patientsinarandomizedcontrolledtrial.CardiolRes. 2013;4:56–63.
23.ShelmadineB,BowdenRG,WilsonRL,BeaversD, HartmanJ.Theeffectsofloweringuricacidlevelsusing allopurinolonmarkersofmetabolicsyndromeinend-stage renaldiseasepatients:apilotstudy.AnatolJCardiol. 2009;9:385–9.
24.StorhaugHM,NorvikJV,ToftI,EriksenBO,LochenML,Zykova S,etal.Uricacidisariskfactorforischemicstrokeand all-causemortalityinthegeneralpopulation:agender specificanalysisfromTheTromsoStudy.BMCCardiovasc Disorders.2013;13:115.
26.GinsbergMH,KozinF,O’MalleyM,McCartyDJ.Releaseof plateletconstituentsbymonosodiumuratecrystals.JClin Investig.1977;60:999–1007.
27.AretiA,YerraVG,NaiduV,KumarA.Oxidativestressand nervedamage:roleinchemotherapyinducedperipheral neuropathy.RedoxBiol.2014;2:289–95.