REVISTA
BRASILEIRA
DE
ANESTESIOLOGIA
OfficialPublicationoftheBrazilianSocietyofAnesthesiologywww.sba.com.br
SCIENTIFIC
ARTICLE
Subarachnoid
meloxicam
does
not
inhibit
the
mechanical
hypernociception
on
carrageenan
test
in
rats
夽
Lanucha
Fidelis
da
Luz
Moura
a,∗,
Silvana
Bellini
Vidor
b,
Anelise
Bonilla
Trindade
b,
Priscilla
Domingues
Mörschbächer
b,
Nilson
Oleskovicz
c,
Emerson
Antonio
Contesini
daUniversidadeFederaldoRioGrandedoSul(UFRGS),PortoAlegre,RS,Brazil
bPostgraduatePrograminVeterinaryScience,UniversidadeFederaldoRioGrandedoSul(UFRGS),PortoAlegre,RS,Brazil cUniversidadedoEstadodeSantaCatarina,Florianópolis,SC,Brazil
dFaculdadedeVeterináriadaUniversidadeFederaldoRioGrandedoSul(UFRGS),PortoAlegre,RS,Brazil
Received21August2013;accepted28October2013
Availableonline2January2015
KEYWORDS
NSAIDs; Carrageenan; Pain;
Spinalcord
Abstract
Backgroundandobjective: Evaluatetheantinociceptiveeffectsofsubarachnoidmeloxicamon themechanicalhypernociceptioninducedbycarrageenaninrats.
Methods:Randomizedcontrolledtrial.EighteenadultmaleWistarratsunderwentacannula implantationintothesubarachnoidspaceandwererandomlydividedintotwogroups:GroupI receivedsalinesolution5L,whileGroupIIreceivedmeloxicam30mg.Themechanical hyper-nociceptionwasinducedbyintraplantarinjectionofcarrageenanandevaluatedusingadigital analgesymeterevery 30minduringa4-hperiod.The resultswererecordedasthe with-drawalthreshold(ing),calculatedbysubtractingthemeasurementvalueaftertreatmentfrom baseline.
Results:Thewithdrawalthresholdmeanvalueswerelowerinthegroupofpatientstreated withmeloxicamoveralltimepointsbetween45and165min,however,therewasnostatistical significance(p=0.835)forthisdifference.
Conclusion:Subarachnoidmeloxicamatadoseof30ganimal−1didnotsuppressthe
mechan-icalhypernociceptioninamodelofinflammatorypaininducedbyintraplantaradministration ofcarrageenaninrats.Thedatasuggestthatotherdosagesshouldbeinvestigatedthedrug effectisdiscarded.
©2014SociedadeBrasileiradeAnestesiologia.PublishedbyElsevier EditoraLtda.Allrights reserved.
夽 WorkconductedatHospitaldeClínicasdePortoAlegre(HCPA),PortoAlegre,RS,Brazil.
∗Correspondingauthor.
E-mail:lanuchamoura@terra.com.br(L.F.L.Moura).
http://dx.doi.org/10.1016/j.bjane.2013.10.020
PALAVRAS-CHAVE
AINE; Carragenina; Dor;
Medulaespinhal
Meloxicamsubaracnoidenãoinibeahipernocicepc¸ãomecânicanotesteda carrageninaemratos
Resumo
Justificativaeobjetivo: avaliarosefeitosantinociceptivosdomeloxicamsubaracnóideosobre ahipernocicepc¸ãomecânicainduzidapelacarrageninaemratos.
Métodos: estudorandômicoecontrolado.DezoitoratosWistar,machosadultos,foram submeti-dosàimplantac¸ãodeumacânulasubaracnóidea,ealeatoriamentedistribuídosemdoisgrupos: oGrupoI(GI)recebeu5Ldesoluc¸ãosalina,enquantoqueaoGrupoII(GII)foramadministrados 30gdemeloxicam,ambospelaviasubaracnóidea.Ahipernocicepc¸ãomecânicafoiinduzida pelainjec¸ãointraplantardecarrageninaeavaliadacomoempregodeumanalgesímetrodigital acada30minutosduranteumperíodode4horas.Osresultadosforamregistradoscomoodo limiarderetirada(g),calculadosubtraindo-seovalordasmensurac¸õesapósostratamentos, dovalorbasal.
Resultados: osvaloresmédiosdodolimiarderetiradaforammenoresnogrupotratadocom meloxicamaolongodetodososmomentosdeavaliac¸ãoentre45e165minutos,contudonão foidemonstradasignificânciaestatística(p=0,835)paraessadiferenc¸a.
Conclusão:aadministrac¸ão subaracnóideadomeloxicamnadosede30 g.animal-1não foi
capazde suprimirahipernocicepc¸ãomecânica emum modelode dorinflamatóriainduzida pelaadministrac¸ãointraplantardecarrageninaemratos.Osdadossugeremqueoutrasdoses sejampesquisadasantesqueoefeitodofármacosejadescartado.
©2014SociedadeBrasileira deAnestesiologia.PublicadoporElsevierEditoraLtda.Todosos direitosreservados.
Introduction
Evidence has shown that besides the known peripheral action nonsteroidalanti-inflammatorydrugs (NSAIDs)have apowerfuleffectonexperimentalpainthatisindependent ofitsanti-inflammatoryeffects.1Inadditiontoits periph-eralinhibitionofprostaglandinsynthesis,acentralactionof NSAIDshasbeensuggestedbyexperimentalstudiesinwhich these drugs demonstrated greater potency by subarach-noidadministrationcomparedtosystemicadministration.2,3 Studieshave shownthatboth cyclooxygenase(COX) forms areconstitutivelyexpressedinthebrainandspinalcordof rats,4withCOX-2thepredominantisoforminthespinalcord dorsal horn.5 Spinal administration of anti-inflammatory drugshasshowntosuppressthereflectionofCfibers,inhibit neuronal sensitizationin the spinal cord dorsal horn, and attenuatelong-terminflammatorypain.2,6---11
Meloxicam is an analgesic and nonsteroidal anti-inflammatorydrug,whichbelongstothephenolicacidclass andhasapreference forCOX-2isoenzyme.12 Unlikemany other NSAIDs, it has high oral bioavailability and a long half-life, althoughnot freefromsideeffects.13 Studiesof meloxicamadministeredbyspinalpathwaysarescarce14---17 anddo notassess itseffects onacuteinflammatory pain. The aimof thisstudywastoevaluatetheantinociceptive powerofsubarachnoidmeloxicamonacutepaininducedby carrageenaninrats.
Materials
and
methods
The experimental protocol was reviewed and approved by the Animal Experimentation Ethics Committee of the
institution.Ratswereindividuallyhousedundercontrolled temperature(21---24◦C)and light---darkcycle of 12h, with foodandwateradlibitumofferedforatleast14days.
(30G×21mm).Finally,theouterportionofthecannulawas fixedtothe skin withsutures. Duringthe periodthat fol-lowedthecannulaimplantation,theanimalswerekeptin individualplastic boxes underthe same conditionsof the previous period. On the day following the cannula place-ment,theanimalswereassessedforneurologicaldeficits. Thosewithneurologicalabnormalitieswereexcludedfrom thestudy.
EighteenWistar rats,weighing300---450gwere success-fullypreparedforthestudyand,onedayafterthecannula placement, they were tested for mechanical hypernoci-ception induced by carrageenan. For this purpose, we usedadigitalanalgesymeter(InsightScientificEquipment Ltda, Ribeirão Preto --- SP, Brazil), according to a previ-ouslydescribedtechnique,19inwhichapressuretransducer equippedwitha7mm2polypropylenetipwasapplied per-pendiculartotherightplantarsurfaceoftheanimals,with alinearlyincreasingpressure.Theequipmentrecordedthe force in grams (g) with a precision of 0.1g. The animal limb stimulation wasrepeated untilobtaining three simi-larmeasurements(thedifferencebetweenthehighestand the lowest value wasless than 10g). Thus, the nocicep-tivebehaviorwasquantifiedbyaveragingthethreevalues expressedingrams,whichrepresentsthepawwithdrawal thresholdtothemechanicalstimulationateachtimepoint. Limbwithdrawalfromcontactwiththetiporshakingand/or lickingbehaviorat thetimeor immediatelyafter stimula-tion(flinch) wasconsidered positiveresponse.Ambulation was considered an ambiguous response; therefore, when it occurred at the time of test application, the test was repeated.
About 30min before starting the assessment, the rats weretransferredtothetestingplace,consistingofacrylic boxes with malleable wire mesh floor, in a quiet room, allowingitsacclimatizationevidenced by cessationofthe grooming and site exploration behavior. Throughout this period,approximatelyfivestimulationsofanimallimbswere performed,inordertoallowtheirfamiliaritywiththe stimu-lusapplied.Subsequently,baselinevaluesfromeachanimal wereestablished.
Afterregistration of baselinevalues,the animalswere manuallyrestrainedandthemetalfragmenttheoccluding thecatheterwasremoved.Next,theanimalswere random-izedintotwogroups.AnimalsinGroupI(GI,n=9)underwent subarachnoidadministrationof 5L salinesolution, while animals in Group II (GII, n=9) received meloxicam 30g diluted in saline to a final volume of 5L, by the same route.Solutionswereadministeredwiththeaidofa Hamil-ton10Lmicrosyringe(701N, HamiltonCompany, Reno ---NV, USA) over a period of 30s, after which, 10L sterile salinewasinjectedtoflushthecatheter.
Immediately after substance administration into sub-arachnoid space, lambda-carrageenan (0.1mL of 2.5% carrageenan) wasinjected into theintraplantar regionof therightlimb,followingthetechniquepreviouslydescribed in the literature.20 The carrageenan injection time was recorded as time-zero (T0), and subsequent evaluations were performed every 30min during the 4h post-drug administrationtoobtainitstemporalprofileof action.All assessments were performed by an investigator who was blindtoanimaltreatment.Becausetheanimalsweretested intheperiodsbeforeandaftertheadministrationofdrugs,
Table1 Meandifference,standarderror,andp-valueof
withdrawalthreshold(g)inratsfromGI andGIIat differ-enttimepointsofhypernociceptioninducedbyintraplantar injectionofcarrageenan.
Timepoints Estimatedmean difference
Standard error
p
30---60min −4.13 3.38 0.914
30---90min −5.60 3.82 0.813
30---120min −7.59 2.96 0.234
30---150min −13.64 4.03 0.055
30---180min −15.30 4.24 0.036
30---210min −23.50 3.68 <0.001
30---240min −27.76 2.53 <0.001
60---90min −1.47 2.42 0.998
60---120min −3.46 2.58 0.872
60---150min −9.51 3.13 0.104
60---180min −11.16 3.67 0.104
60---210min −19.37 2.71 <0.001
60---240min −23.63 2.88 <0.001
90---120min −1.99 2.84 0.996
90---150min −8.04 2.67 0.111
90---180min −9.70 3.35 0.136
90---210min −17.90 2.71 <0.001
90---240min −22.16 2.99 <0.001
120---150min −6.06 2.41 0.254
120---180min −7.71 3.81 0.497
120---210min −15.92 2.22 <0.001
120---240min −20.18 2.80 <0.001
150---180min −1.65 3.37 1.000
150---210min −9.86 2.60 0.025
150---240min −14.12 3.02 0.004
180---210min −8.21 2.36 0.046
180---240min −12.47 3.03 0.013
210---240min −4.26 2.48 0.677
the results were recorded asthe withdrawal threshold (g),calculatedbysubtractingthemeasurementvalueafter treatmentsfromthebaselinevalue,andthesevalueswere compared.
Dataare expressed as mean±standard deviation. The withdrawalvalueswerecomparedusingvariance analy-siswithrepeatedmeasuresandtwofactors,withGIorGII groupasthefixedfactorandthetime(every30min)asthe repetitionfactor.Foranalysis,anunstructuredcorrelation matrix between timepointswassupposed. Analyses were performedwithSASsoftwareversion8.0forWindows,and thelevelofsignificancewasdeterminedatp<0.05.
Results
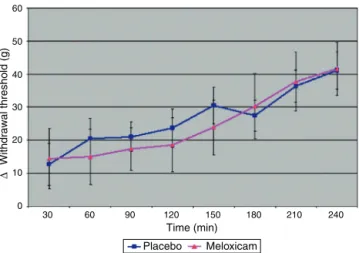
A mean increase of the withdrawal threshold occurred overtime,withstatisticallyhighervaluesinassessmentsat 210and240mincomparedtoothertimepoints(p<0.05). Thereweredifferencesbetweenothertimepoints,always with a mean increase of withdrawal threshold in the courseoftheexperiment,asshowninTable1.
60
50
40
30
20
10
0
30 60 90 120 150 180 210
∆
Withdrawal threshold (g)
Time (min)
240
Placebo Meloxicam
Figure1 Meanvaluesofwithdrawalthreshold±standard
deviation(g)indifferenttimepointsofmechanical
hypernoci-ceptioninratsafterSAadministrationofsalinesolution(GI)or
meloxicam(GII).
significant(p=0.835)forthisdifference(Fig.1).Mean dif-ferencesinthewithdrawalthresholdoccurredbetween timepointswithineachgroup(p<0.001).
During antinociceptionevaluation, tworats,both from GI,showedclinicallydifferentiatedresponses after induc-tion of hyperalgesia. These animals presented with eye discharge,significantprostration,withvocalizationatrest and reluctant to rest the limb subjected to carrageenan application, suggesting the presence of severe pain. The antinociceptive evaluation for these rats showed a high degreeofdifficultycomparedtoothers,astheanimalsdid notallow force to beexertedagainst theplantar surface during measurements. In these cases, the animals raised theiraffectedlimb,followingthetipmovement,not exert-ingpressureresistance.Asaresult,theseanimalsshowed highlevelsofwithdrawalthreshold,whichwerenot consis-tentwithwhatcouldbeobservedclinically.
Discussion
TheeffectivenessofconventionalNSAIDs,COX-2inhibitors, andmonoclonalanti-prostaglandinE2asanti-inflammatory agents in experimental models using carrageenan is well described in the literature.20---22 The actions were tradi-tionally assigned to peripheral prostaglandin inhibition, which play an important role in nociceptor sensitization at the site of injury,23 as carrageenan intraplantar injec-tioninducesa significantincrease ofCOX-2expression,as wellasprostaglandinE2production.24However,therelative amountsofeachisoformexpressedindifferenttissuesvary andmaybemodulatedinpathologicalconditions.Thus,in contrasttootherorgans,therat’snormalbrain,aswellas the spinal cord,expressesmore COX-2than COX-1,25 and data confirm its role in the sensory processing of pain.26 Thus,aseriesofexperimentalevidencecurrentlysuggests thatNSAIDsexerttheiranalgesicactionalsobyactivityon the centralnervous system, in additiontoits well-known peripheralaction.25
Severaldrugshavebeenadministeredbyspinalroutein an attempt to prove such a mechanism, and the lack of
meloxicamconsistenteffectsonspinalnociceptioninduced intheexperimentalmodelusedcontradictsthefindingsof other authors whoreported antinociceptive activity after spinal use of other NSAIDs.2,14,15,20,27---32 It is noteworthy, however,thatalthoughthereareavarietyofstudies eval-uating the antinociceptive power of COX-2 administered in the subarachnoidspace, studies specifically investigat-ingtheeffectsofmeloxicamarestillscarce.Thesestudies havefoundaninhibitoryeffectonthewind-upphenomenon invitro,33 aswell ason nociception induced by capsaicin or formalin.34 Additionally, a synergistic analgesic effect with morphine was found after its SA administration in animals with experimental visceral pain.15 In a previous study,17adosesimilartotheoneusedinthepresentstudy (30ganimal−1)was usedto investigate theantiallodynic effects of SA meloxicam. The authors, however, used an experimentalmodelof neuropathicpainin diabeticmice, differingfromthisstudyinflammatorypainmodel.However, the drug effects were demonstrated with the dose used, whichdid notoccur in thisstudy. Observingcarefully the datashowninFig.1,onecanseethatthegroupreceiving meloxicam showed mean values of withdrawal thresh-oldlowerthan thoseshown by the group receivingsaline solutionduringalltimepointsbetween45and165min.
The intraplantar carrageenan inflammation is charac-terized by a biphasic behavior with respect to swelling. Theearlyphase(0---1h)hasbeenattributedtotherelease ofhistamine,5-hydroxytryptamine,andbradykinin,sothe effectivenessofNSAIDsinthisperiodhasbeenquestioned. Inthelatephase(1---6h),ontheotherhand,ahigh produc-tionofPGshasbeenverified.35However,hyperalgesiaseems todevelopinparallelwiththeincreaseofCOX-2spinal lev-els,anditspeakonly occurswithin4hafter carrageenan injection.36LookingatFig.1,however,onecanseethatthe effectsofmeloxicamonhyperalgesiaoccurredpreciselyin theperiodpriortothe4hofassessment,withsignificantly highervalues of withdrawal thresholdafter 165min of administration,whichdiffersfromsuchstatements.
However, this response curve behavior has also been observed in studies using carrageenan model, which reportedanti-hyperalgesicthermaleffectswithSA adminis-trationofSC58125---aselectiveCOX-2inhibitor---onlyduring the first170min of evaluation.37 As thermal hyperalgesia has been shown to be similarly mediated by the action ofspinalCOX-2,38 studies thatcharacterizethepatternof spinalCOX-2expression incarrageenan-induced inflamma-tion,hithertoseeminglynonexistent,mayhelptoelucidate suchobservations.Asignificant increase inthe hypernoci-ceptionintensity,characterizedbyincreasedwithdrawal threshold,especiallyinassessmentsat210and240min,was observedinbothexperimentalgroups. Thesefindingsmay beexplained by other authors who report that the repe-titionof mechanicalstimulationcanproduceanincreased sensitivityofthe stimulated area.39 Thus,the administra-tion of meloxicam also failed to prevent the increase of hypernociceptionovertime.
suchascontinuousinfusion techniques or itsassociation withopioids.15 The need todetermine a dose that shows consistenteffects,oreventheabsenceofsucheffects,was basedontheextrapolationofresultsobtainedinthese stud-iesand their suitability to the needs of this work. Thus, basedonprevious studiesthat showedsatisfactoryresults using30-ganimal−1ofsubarachnoidmeloxicamon exper-imental neuropathic pain in diabetic animals,17 a similar dose wasrecommended inordertoobserveitseffects on inflammatoryhyperalgesia.
Neuropathic pain, however, is a complex syndrome involving inflammatory and immune theories yet to be understood,andwhosehyperalgesiaresultsfromboth neu-ralandnon-neuraltissueinvolvementandisassociatedwith AandA␦fibersactivation.40However,thenociceptive sys-temcomplexityhasshownthatdifferentsensorypathways are activated after minimal changes in the nature of a painful process,41 leading to believe that different doses ofthesame drugmayberequiredfor suppressionof pain ofdifferentorigins.Sucha possibilitycan bereadilyseen inthepresent study,asadose capableofcontrolling neu-ropathichyperalgesia did notachieve the sameresults as thatof inflammatory origin, thus demonstrating the need forfurtherstudieswithdifferentdosesofthedrug.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorsthankCNPq/CAPESforfundingtheresearch.
References
1.VinegarR,TruaxJF,SelphJL,etal.Pathwayto carrageenan-induced inflammationinthehind limboftherat. Fed Proc. 1987;46:118---26.
2.MalmbergAB,YakshTL.Antinociceptiveactionsofspinal non-steroidalanti-inflammatoryagentsontheformalintestinthe rat.JPharmacolExpTher.1992;263:136---46.
3.MalmbergAB,YakshTL.Hyperalgesiamediatedbyspinal gluta-mateorsubstancePreceptorblockedbyspinalcyclooxygenase inhibition.Science.1992;257:1276---9.
4.BrederCD, DewittD,KraigRP.Characterization ofinducible cyclooxygenaseinratbrain.JCompNeurol.1995;355:296---315. 5.BurianM,GeisslingerG.COX-dependentmechanismsinvolved intheantinociceptiveactionofNSAIDsatcentralandperipheral sites.PharmacolTher.2005;107:139---54.
6.Beiche F, Scheuerer S, Brune K, et al. Up-regulation of cyclooxygenase-2mRNAintheratspinalcordfollowing periph-eralinflammation.FEBSLett.1996;390:165---9.
7.BianchiM,PaneraiAE.Thedose-relatedeffects of paraceta-molonhyperalgesiaandnociceptionintherat.BrJPharmacol. 1996;117:130---2.
8.BustamanteD,PaeileC,WillerJC,etal.Effectsofintrathecal orintracerebroventricularadministrationofnonsteroidal anti-inflammatorydrugsonaC-fiberreflexinrats.JPharmacolExp Ther.1997;281:1381---91.
9.HerreroJF,ParradoA,CerveroF.Centralandperipheralactions oftheNSAID ketoprofenonspinal cordnociceptive reflexes. Neuropharmacology.1997;36:1425---31.
10.McCormackK.Non-steroidalanti-inflammatorydrugsandspinal nociceptiveprocessing.Pain.1994;59:9---43.
11.WillingaleHL,GardinerNJ,McLymontN,etal.Prostanoids syn-thesizedbycyclo-oxygenaseisoformsinratspinalcordandtheir contributiontothedevelopmentofneuronalhyperexcitability. BrJPharmacol.1997;122:1593---604.
12.Leal LB, Silva MCT, Bedor DCG, et al. Desenvolvimento de testededissoluc¸ãoparaomeloxicamutilizandooplanejamento fatorial:estudocomparativodeprodutosindustrializadosx pro-dutosmagistrais.RevBrasFarm.2008;89:160---3.
13.TurnerPV,ChenHC, TaylorWM.Pharmacokinetics of meloxi-caminrabbitsaftersingleandrepeatoraldosing.CompMed. 2006;56:63---7.
14.Pinardi G, Sierralta F, Miranda HF. Atropine reverses the antinociception of nonsteroidal anti-inflammatory drugs in the tail-flick test of mice. Pharmacol Biochem Behav. 2003;74:603---8.
15.PinardiG,PrietoGC,MirandaHF.Analgesicsynergismbetween intrathecalmorphineandcyclooxygenase-2inhibitorsinmice. PharmacolBiochemBehav.2005;82:120---4.
16.TakedaK,SawamuraS,TamaiH,etal.Roleforcyclooxygenase 2inthedevelopmentandmaintenanceofneuropathicpainand spinalglialactivation.Anesthesiology.2005;103:837---44. 17.Kimura S, Kontani H. Demonstration ofantiallodynic effects
of the cyclooxygenase-2 inhibitor meloxicam on estab-lished diabetic neuropathic pain in mice. J Pharmacol Sci. 2009;110:213---7.
18.Yaksh TL, RudyT. Chroniccatheterization ofthe spinal sub-arachnoidspace.PhysiolBehav.1976;17:1031---6.
19.Vivancos GG, Verri WA Jr, Cunha TM, et al. An electronic pressure-meternociceptionpawtestforrats.BrazJMedBiol Res.2004;37:401---7.
20.FrancischiJN,ChavesCT,MouraAC,etal.Selectiveinhibitors ofcyclo-oxygenase-2(COX-2)inducehypoalgesiainaratpaw modelofinflammation.BrJPharmacol.2002;137:837---44. 21.Zhang Y, Shaffer A, Portanova J, et al. Inhibition of
cyclooxygenase-2 rapidly reverses inflammatory hyperalgesia and prostaglandin E2 production. J Pharmacol Exp Ther. 1997;283:1069---75.
22.Riendeau D, Percival MD, Brideau C, et al. Etoricoxib (MK-0663): preclinical profile and comparison with other agents thatselectivelyinhibitcyclooxygenase-2.JPharmacolExpTher. 2001;296:558---66.
23.KellyDJ,AhmadM,BrullSJ.PreemptiveanalgesiaI: physiolog-icalpathwaysandpharmacologicalmodalities.CanJAnaesth. 2001;48:1000---10.
24.NantelF,DenisD,GordonR,etal.Distributionandregulation ofcycloocygenase-2incarrageenan-inducedinflammation.BrJ Pharmacol.1999;128:853---9.
25.Martin F, Fletcher D, Chauvin M, et al. Constitutive cyclooxygenase-2isinvolvedincentralnociceptiveprocesses inhumans.Anesthesiology.2007;106:1013---8.
26.VanegasH,SchaibleHG.Prostaglandinsandcyclooxygenasesin thespinalcord.ProgNeurobiol.2001;64:327---63.
27.Wang BC, Hiller JM, Simon EJ, et al. Analgesia follow-ing subarachnoid sodium ibuprofen in rats. Anesthesiology. 1992;79:856.
28.WangBC,LiD,HillerJM,etal.Theantinociceptiveeffectof S-(+)-ibuprofeninrabbits:epiduralversusintravenous adminis-tration.AnesthAnalg.1995;80:92---6.
29.Parris WC, Janicki PK, Johnson B Jr, et al. Intrathecal ketorolactromethamineproducesanalgesiaafterchronic con-strictioninjuryofsciaticnerveinrat.CanJAnesth.1996;43: 867---70.
31.Miranda HF, Pinardi G. Lack of effect of naltrexone on the spinal synergism between morphine and non steroidal anti-inflammatorydrugs.PharmacolRep.2009;61:266---74. 32.Eisenach JC, Curry R, Tong C, et al. Effects of
intrathe-cal ketorolac on human experimental pain. Anesthesiology. 2010;112:1216---24.
33.Lopeza-GarciaJA, Laird JM. Central antinociceptive effects of meloxicam on rat spinal cord in vitro. Neuroreport. 1998;9:647---51.
34.SantosAR,VedanaEM,DeFreitasGA.Antinociceptiveeffectof meloxicam,inneurogenicandinflammatorynociceptivemodels inmice.InflammRes.1998;47:302---7.
35.Di Rosa M, Sorrentino L. Some pharmacodynamic proper-ties of carrageenin in the rat. Br J Pharmacol. 1970;38: 214---20.
36.HilárioMO,TerreriMT,LenCA.Nonsteroidalanti-inflammatory drugs:cyclooxygenase2inhibitors.JPediatr.2006;82:S206---12. 37.Dirig DM, Isakson PC, Yaksh TL. Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. JPharmacol Exp Ther. 1998;285:1031---8.
38.YamamotoT,Nozaki-TaguchiN.Roleofspinalcyclooxygenase (COX)-2onthermalhyperalgesiaevokedbycarageenan injec-tionintherat.Neuroreport.1997;8:2179---82.
39.LeBarsD,GozariuM,CaddenSW.Animalmodelsofnociception. PharmacolRev.2001;53:597---652.
40.Kraychete DC,GozzaniJL, Kraychete AC.Dor neuropática ---aspectosneuroquímicos.RevBrasAnestesiol.2008;58:492---505. 41.MillanMJ.Theinductionofpain: anintegrativereview.Prog