Inuene of Chemial Struture on the Mesomorphi
Behaviour of 3,5-Disubstituted 1,2,4-Oxadiazoles
S. Torgova 1;3
, L. Karamysheva 1;3
, and A. Strigazzi 2;3
1
FSUE"SRC"NIOPIK"(OrganiIntermediates&DyesInstitute),
B.Sadovaya1/4, Mosow 103787,Russia
2
DipartimentodiFisia,andIstitutoNazionalediFisiadellaMateria (INFM),
PoliteniodiTorino,C.Duadegli Abruzzi24, I-10129Torino,Italy
3
JointLaboratory ofOrientationally Ordered Media(OOM-Lab),
C.Dua degliAbruzzi24,I-10129 Torino,Italy
Reeivedon15January,2002
Theorrelationbetweenhemialstrutureandmesomorphipropertiesisoneofthemost
impor-tant problems inliquid rystals siene. 3,5-Disubstituted 1,2,4-oxadiazoles are veryonvenient
model-ompoundsforstudyingthedependeneoftheLCproperties onthemoleulardesign. The
transitiontemperaturesanddieletri propertiesof1,2,4-oxadiazolesdependsigniantlybothon
the position of the substituents withrespet to the heteroyle and ontheir donor or aeptor
features.
I Introdution
The orrelationbetweenhemialstruture and
meso-morphi propertiesisoneofthemostimportant
prob-lems in liquidrystals(LC)siene. Knowledgeabout
the inuene of dierent strutural elements of the
moleulesonthephysio-hemialharateristisof
me-somorphi organiompounds allowshemiststo
syn-thesize LC with the requiredproperties. The idea to
use nonsymmetrialheteroylesasmodel ompounds
fortheinvestigationofhemialstrutureinuene on
the mesomorphi behaviour ame to us many years
ago. Therstheteroyliompoundswerederivatives
of imidazo[2,1-b℄-1,3,4-thiadiazoles [1℄. These
prati-ally planar and rigid heteroaromati systems with
two ondensed heteroyles, whih have dierent
-onjugations, have never been used before as a
frag-mentofLCmoleules. Themostinterestingresultwas,
thatthemesomorphipropertiesofnewmesogenswere
stritly dependent not only on the nature of the
sub-stituents,but alsoontheir position in thethiadiazoli
or imidazoli part of the moleule. 3,5-Disubstituted
1,2,4-oxadiazolesappearedtobeevenmoreonvenient
model-ompounds for studying the dependene of LC
II Experimental
The synthesis of imidazo[2,1-b℄-1,3,4-thiadiazoles has
beendesribedinthepaper[1℄. Thesynthesisof
1,2,4-oxadiazole derivatives was arried out aording to a
previouslyreportedmethod [2℄.
Transition temperatures were determined using a
Mettler FP-51 thermoontroller and Leitz polarizing
mirosopeonnetedtoaGrundig/Polaroidreording
system. The dierential sanning alorimetry (DSC)
datawere olletedbymeans ofPerkin-Elmer
appara-tus.
NMR spetrawere reordedon a BrukerWM-250
spetrometer, by using CDCl
3
as a solvent.
Satisfa-toryelementalanalyseswereobtainedforallnew
om-pounds.
III Results and disussion
We have synthesized and investigated a lot of
imida-zothiadiazoliderivatives. InTable1someexamplesof
theompounds synthesized aregiven. Letus ompare
thetransitiontemperaturesand thephasesequene of
thesederivatives.
Compounds 1and2haveaverysmalldierenein
stru-Replaing the benzene ring with a ylohexane one
(ompounds1and3),weanobserveonlythenemati
phase. Thepreseneofaylohexaneringin the
thia-diazoli partof themoleuleauses theappearaneof
anematiphaseonly,whereasthesameringinthe
imi-dazolipartofthemoleuleleadstobothasmetiand
anematiphase. Itisneessarytounderline,thatthis
behaviouris independentof theylohexane ring
on-netion, whetheritisdiretly onnetedtotheentral
heteroylifragment(ompounds3,5and4),orforms
part of the phenylylohexyl fragment (ompounds 7
and6).
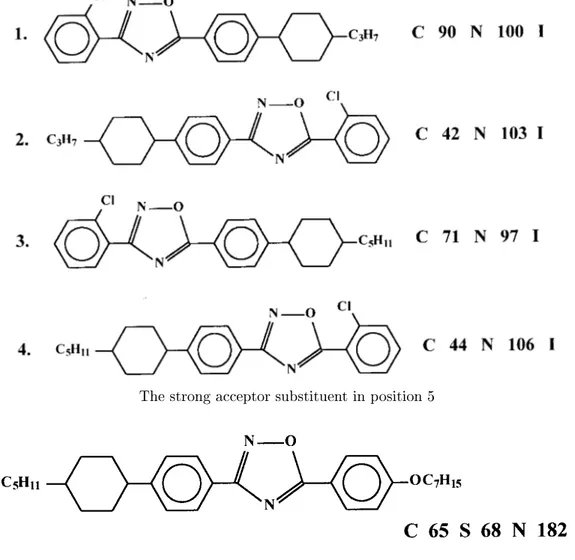
Table3. Inreasingofthemoleularpolarizationbyintrodutionofthepolargroupsinposition3and5ofoxadiazole
inreasesthemeltingpointsofLC.
Weakaeptorsubstituentinposition3
Thestrongaeptorsubstituentin position 5
Table4. Inueneofthestrongaeptoranddonorsubstituentsandyloxexanefragment.
Strongaeptorsubstituent
Theombinationofastrongdonorandaeptorsubstituents
Theinueneoftrans-1,4-ylohexanefragmentonthetypeof themesophase
The ombination of a strong donor substituent in
position 3 and astrong aeptor in position 5 makes
the dierene in the dieletri anisotropy, ompared
with the\reversed"struture,morepronouned
(om-pounds 4and 3,Table4). Mesogenswith a
trans-1,4-harater as ompared with biphenyl analogs
(om-pounds 6 and 5, Table 4). With the help of a
ross-ouplingreation,whih weused forthe rsttime for
the synthesis of this lass of ompounds, we had the
Compound 1 possesses both smeti and nemati
phases. Compound2{hasabentmoleularshapeand
exhibitsonlyanematiphase,thethirdompoundhas
nomesophaseatall(Table5). Thelastompoundwas
obtained in very small yield (less then 10%). We
as-sume thatitwasduetosteridiÆulties.
Pyridine-ontaining oxadiazoles were also
synthe-sized, primarily with the purpose of heking the
or-relationbetween thethermal and dieletriproperties
ofnewLCandthepositionofthenitrogenatominthe
pyridine ring (ortho-,meta- and para-with respet to
theoxadiazoliring,Struture4inTable5).
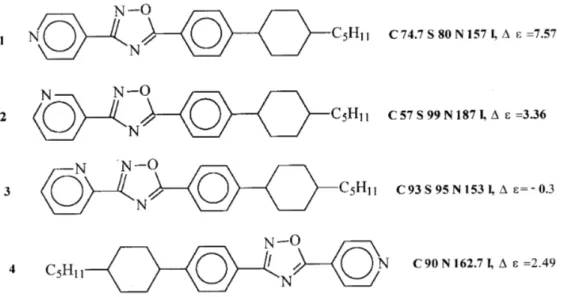
InTable6thedatafromthethermalanddieletri
measurements for the pyridine ontaining ompounds
arepresented.
Table6. Dependene ofthetransitiontemperaturesof1,2,4-oxadiazoleswithpyridinesubstituentsontheposition
of thenitrogenatomin thepyridine ring.
Liquid rystals with meta-pyridine substituents
(ompound2)havethelargestmesophaseinterval,with
para-pyridine (ompound 1) they have an
intermedi-atemesophaserange,asomparedwithortho-pyridine
(ompound 3). Moreover, oxadiazoles with
para-pyridine havearatherlargedieletrianisotropyvalue
(7.57), with meta-pyridine the dieletri anisotropyis
around half this value(3.36), and withortho-pyridine
thevalueisevennegative({0.3).
The inuene of the position of the pyridine
sub-stituentsisalsoevident: whenitisinthediazolipart,
bothsmeti andnemati phasesare present. The
in-trodutionofapyridinesubstituentintheoxazolipart
of the moleule leads to the presene of the nemati
phaseonly(ompareompounds1and 4).
It is neessaryto pointout thepeuliar behaviour
of alkyl-substituted oxadiazoles. In order to derease
the melting points of oxadiazoli derivatives we have
designed and synthesized a series of nonsymmetri
three yles ontaining moleules where one of the
two substituents is an alkyl-group in the C
3 or C
5
-positions of the 1,2,4-oxadiazole,whereasthe other is
fragment. An alkyl-substituentin position-3leadstoa
highermeltingpointandamoresmetogeniharater,
thanin position-5(Table7).
The most interesting and unexpeted results were
obtainedfromtheDSCanalysisofthealkyl-substituted
oxadiazoles. Thephasetransition enthalpiesshoweda
peuliarbehaviour. Inthe3-alkylontainingseriesthe
peakofisotropitransition ismuh greaterthanthose
for all other transitions, inluding the peak of
melt-ing from rystal to mesophase. In ontrast,
5-alkyl-1,2,4-oxadiazolesdemonstratedthetraditional
orrela-tion betweenenthalpy peaks: the maximumenergy {
for the rystal-smeti transition, the minimum { for
thenemati-isotropitransition.
\Banana-shaped"moleules on thebase of
oxadia-zoles were also synthesized and investigated [8℄. Our
aim was to synthesize \banana-shaped" esters,
on-taining asymmetri 1,2,4-oxadiazole as a entral unit
(Figure2). Thediretlinkagebetweentheheteroyli
groupandthephenylringsensuresabendof141inthe
All new ompounds synthesized exhibited a wide
mesomorphiinterval. ThespontaneouspolarizationP
in the smeti, and even in the low temperature
ne-mati phasesof this ompound were measuredby the
method ofrepolarizationurrent,byusingtriangle
im-pulses. ThevalueofPturnedouttobeoftheorderof
10 {20 nC/m 2
. However,at presentit is impossible
to unambiguouslyonlude,thattheonlyontribution
toPmeasuredisthatgivenbythepolarizationofsuh
LC phases. This will be thesubjetof ourfurther
in-vestigations.
Figure 2. \Banana-shaped"esters, ontaining asymmetri
1,2,4-oxadiazole(I)asaentralunit.
IV Conlusion
A variety of mesogeni 3,5-disubstituted
1,2,4-oxadiazoles have been synthesised. The results
pre-sentedhereshowthatthisnewlassofliquid-rystalline
ompoundsis veryusefulforinvestigatingthe
orrela-tion between the hemial struture of mesogens and
Aknowledgements
ThisworkwaspartiallysupportedbytheEuropean
Community in the frame of INCO Copernius
Con-ertedAtion\Photoom",under ContratNo.
IC15-CT98-0806,andin theframeoftheBRITE-EuramIII
TNLCPhotonet. Twooftheauthors(S.I.T.andA.S.)
fullyaknowledgethesupportoftheSientiand
Or-ganizingCommitteeof"5thIbero-AmerianWorkshop
onComplex Fluids and their Appliation", whih
en-abledthem toattendtheConferene.
Referenes
[1℄ A.V.Ivashhenko,S.I.Torgova,L.A.Karamysheva,and
A.G.Abolin,Liq.Cryst.7,475(1990).
[2℄ L.A.Karamysheva,S.I.Torgova,I.F.Agafonova,andR.
Ch.Geivandov,Mol.Mater.4, 289(1994).
[3℄ L.A. Karamysheva, S.I. Torgova, I.F. Agafonova, and
N.M.Shtikov,Mol.Cryst.Liq.Cryst.160,217(1995).
[4℄ O.Franesangeli, L.A.Karamysheva,S.I.Torgova, A.
Sparavigna, and A. Strigazzi, Proeedings of SPIE,
3319,139(1997).
[5℄ L.A. Karamysheva, I.F. Agafonova, and S.I. Torgova,
Mol.Cryst.Liq.Cryst.332,407(1999).
[6℄ L.A.Karamysheva,I.F.Agafonova, S.I. Torgova, B.A.
Umanskii,andA.Strigazzi,Mol.Cryst.Liq.Cryst.364,
547(2001)
[7℄ Comprehensive Heteroyli Chemistry, Eds. A.R.
Ka-trizkyandCh.W.Rees,PergamonPress,6,378(1984).
[8℄ S.I.Torgova,L.A.Karamysheva,T.A.Geivandova,and
A.Strigazzi,Mol.Cryst.Liq.Cryst.365,99(2001).
[9℄ K.J.K.Semmler,T.Y.Dingemans,andE.T. Samulsky,