Lyotropi Ferronemati Liquid Crystals Based on
New Ni, Cu and Zn Ioni Magneti Fluids
C. Y. Matuo 1
,F. A. Tourinho 2
, M. H. Souza 2
,
J. Depeyrot 3
, and A. M. FigueiredoNeto 4
1
Departamentode Fsia,UniversidadeFederaldeSantaCatarina,
CaixaPostal476, 88040-900,SC,Brazil.
2
Institutode Qumia,Universidadede Braslia,
CaixaPostal 04478,70919-970, Braslia,DF,Brazil.
3
Instituto deFsia,UniversidadedeBraslia,
CaixaPostal 04455,70919-970, Braslia,DF,Brazil.
4
Institutode Fsia, UniversidadedeS~aoPaulo,
CaixaPostal 66318,05315-970, S~aoPaulo,SP,Brazil
Reeivedon26November,2001
ThepropertiesoflyotropiferronematiliquidrystalsbasedonnewNi,CuandZnionimagneti
uids are disussed. TheeÆienyofthesenewferrouidsinthelyotropinematiliquidrystal
dopingisveriedandomparedwiththeonventionalsurfatedferrouidlyotropidoping. Itwas
observed that the strutural harateristis of the lyotropis and ferrouids determine the good
formationoftheferronematis.
I Introdution
Liquidrystalsanbeoriented[1℄byeletriand
mag-netieldsorbyshearstress. Sinetheanisotropipart
of the diamagneti suseptibility of these materials is
small, high magneti elds, of about 10 kG, are
ne-essaryto orienttheliquid rystals. In1970, Brohard
and deGennes[2℄ proposed atheory ofmagneti
sus-pensionsin whih liquid rystalsould be doped with
smallmagnetigrains. Theouplingbetweenthe
mag-netigrainsandtheliquidrystallinematrixismainly
frommehanialoriginandthisouplingisresponsible
for the orientation proess [2℄. The doping of liquid
rystalswithferrouids[3,4℄(abovearitial
onen-tration) redues the magneti eld required to orient
theliquidrystalsbyafator10 3
.
Ferrouids ormagneti uids are olloidal
suspen-sions of small magneti grains (typial dimension 10
nm)dispersedinaliquidarrier. Twodierenttypesof
ferrouidsareavailable: surfatedand ioniferrouid.
Thelastoneisalsonamedeletridoublelayered
mag-netiuids(EDL-MF)[5℄. Inthesurfatedferrouids,
the grains (usually Fe
3 O
4
) are oated with surfatant
agents(amphiphilimoleules) topreventtheir
ou-lation (steri repulsion: physial barrier) [4℄. Inpolar
to formanexternalhidrophililayer. Inioni
ferrou-ids, the magneti grains (usually -Fe
2 O
3 ; MnFe
2 O
4 ;
CoFe
2 O
4
) are eletrially harged to keep the olloid
stable (eletri repulsion) [6, 7℄. The ioni itrated
maghemite (-Fe
2 O
3
) has both harateristis (steri
andeletrostati repulsion)topreventtheaggregation
ofthemagneti grains. Whenaferrouidishighly
di-luted in a solvent, it beame unstable and the grains
oulate.
Nematiliquidrystalsdopedwithferrouidshave
beenalled [2℄ferronemati liquid rystals. Following
de Gennes' preditions, many experiments were
per-formed with thermotropi and lyotropi ferronemati
and ferroholesteri (holesteri liquid rystal doped
with ferrouid) liquid rystals. The attainment of a
thermotropi ferronemati isaverydeliate task, due
tothelowsolubilityofthemagnetiuidsintheliquid
rystalline matrix. Usually, theliquid rystal and the
magnetiuidseparateandahomogeneoussolution(as
afuntion of time)is not ahieved. The rst
suess-ful observation of the marosopi olletive behavior
ob-iment,however,the magnetigrainswere muh bigger
thanthat presentin atualferrouids: theyused
mag-neti grains with typial dimensions of mirons. On
the otherhand, thedoping oflyotropiliquid rystals
(mixture of amphiphili moleules and a solvent,
usu-allywater[1℄)withwaterbasesurfatedferrouidswas
doneforthersttimein1979byLiebertandMartinet
[9℄,andsinethenthismethodhasbeenused[10-14℄to
investigatethephysial-hemialpropertiesof
lyotrop-is. Inthemostofthese experimentsthelyotropi
liq-uid rystals were doped with a water base surfated
ferrouidfromFerrouidis Corp..
Colletive behaviorof the liquidrystalline matrix
wasobserved with magneti elds of about 5 G. The
aggregation of thegrains,in thepresene of magneti
elds, forming needles of about 10 m long wasalso
observed[10℄. Depletionlayers,i.e., regionswhere the
magnetigrains aresegregateddue totopologial
on-gurations ofthediretor, [2℄ wereobserved[10℄.
Fol-lowingdeGennes'predition,theminimum
onentra-tions(C
m
)offerrouidmagnetigrainsrequiredto
ori-entnemati liquidrystalsweredeterminedinsamples
dopedwithsurfatedferrouid[11℄andioniferrouid
ofCoFe
2 O
4
[15℄. TheknowledgeofC
m
isimportantin
pratial experiments in orderto preservemostof the
physialpropertiesoftheliquidrystallinesystems.
Dynami proesses in lyotropiferronematis were
also studied [16, 17℄ using pulsed magneti elds. In
these studies, it was veried that there is a dierent
dynamibehaviorbetweenferronematisandusual
ne-matis when samplesare subjetedto magnetields.
In usual nematis the relaxationtime is proportional
toH 2
[18℄andinferronematistherelaxationtimeis
proportionalto H 1
.
Due to the new features of ferronematis, mainly
theirremarkableresponseto lowmagnetields, after
Brohard and de Gennes' paper [2℄, other theoretial
approahesto theeld-diretorouplingproblemwere
done[19,20,21℄.
Sine ferronematis (and ferroholesteris)
onsti-tute anew lassof omplex magnetiuids with
aa-demi and tehnologial interests, it is important to
knowwhattypeofferrouidismoreeÆienttoprodue
ferronematis. Thetype offerrouidused in the
dop-ingproessdependsonthephysisweintendtostudy:
low or high magneti eld-diretor oupling, slow or
fast response to external agents, and soon. Reently
new ioni ferrouids (EDL-MF) based onNi, Cu and
Zn ferritesweresynthesized [5,22℄.
In this paperwe disuss the doping of alyotropi
nemati liquid rystalwith these newferrouids. The
properties of these new ferronematis are ompared
using dierent types of ferrouids. The eÆieny of
eah ofthese newferrouidsin onstitutinga
ferrone-matiisdisussed.
II Experimental setion
II.1 Materials
Thelyotropinemati liquidrystalused isa
mix-ture of potassium laurate (28.74 weight%), deanol
(6.64 wt%) and water (64.62 wt%), with an
uniax-ial alamiti nemati phase N
C
[23℄ between 12 and
35 o
C. The experiments are performed at 22 o
C. The
nemati phase is identied by means of optial
mi-rosopy,onosopyandX-raydiration.
Theioniwaterbasedferrouidsusedforthedoping
havemagneti grains withameandiameter of8.4 nm
(CuFe
2 O
4
),4.4 nm(NiFe
2 O
4
)and 6.3nm(ZnFe
2 O
4 ),
andmagnetization at saturationof 135G, 279G and
0G, respetively. The ferrouids used were prepared
byhemialsynthesisproess. Copper,NikelandZin
[5, 22℄ spinel oxide grains are prepared by
ondensa-tionmethodfromhemialreationamongaqueous
so-lutions of metal mixtures in alkaline medium. Grain
nature and size are xed by opreipitation step. As
farasthemolarratioisonerned,thebestinitial
on-dition for allsample preparedorrespondsjust to the
ferritestoihiometry,i.e.,0:33. Inaddition,the
parti-ular base whih is used, the pH and the temperature
are extremely important in the synthesis. The best
temperature range for the synthesis is around 100 o
C.
Usually a base exess is needed due to the aidity of
the initial mixture. The reagent addition proedure,
inludingthewayandspeedofmixing,atsdiretlyto
determinetheaveragevalueof thegrainsize. Thene
grains are obtained by poring the mixture as quikly
aspossible into the base medium under vigorous
stir-ring. Veryhigh ionistrength, as well asbase exess,
are very important in the synthesis of nikel ferrite
grains. Finally, after the magneti grains being
syn-thesizedtheywerewashedinaidmediumusinga
fer-rinitrate solution at boiling temperature in order to
promotethehemialsurfaestabilization. Verystable
and onentrated magneti uids are obtained, after
thehemialsurfaestabilizationstep,bydispersionin
aid(alkaline)medium with suitableounterions, like
nitrateorperhlorate(tetramethylammonium ation).
Ferrouidsusedinthis studyareaidferrouids,i. e.,
the grains are positively harged. The
harateriza-tion of the ferrouids were done [5, 22℄ by eletroni
measure-We used onentrations of magneti grains above
the minimumonentrationrequired to orient the
ne-matiliquid rystals[11, 15℄in the preparationofthe
ferronematis. The doping was done by arefully
di-luting aonentratedsolutionof ferrouid,whihwas
thenintroduedintoagivenvolumeofnemati witha
mirosyringe. The uids were mixed by smooth
stir-ring. Following this proess, without any
preipita-tion of the magneti grains, theferronemati samples
present(71)10 13
grains/m 3
.
II.2 Methods and tehniques
Oneof the tehniques used is the rossed
polariz-ersoptial mirosopy. The experimentonsists in to
observethe ferronemati samples in apolarizing light
mirosope. The ferronemati sample is enapsulated
insideretangularglassmiroslides(fromVitro
Dynam-is) with the following dimensions: 25 mm (length),
4mm(width)and400m(thikness). Thesamplesare
observedbeforeandaftertheappliationofamagneti
eld,andthetexturesarereordedwithaCCDamera
oupled in the mirosope. A magneti eld ofabout
200Ganbeapplied tothesampleonthemirosope
stage. IntheaseofN
C
samples,thediretor(optial
axis)orientsparalleltotheapplied magnetield[12℄.
Themirosopestageallowstheobservationofthe
fer-ronematitexturewithdierentrelativeorientationsof
thediretor with respet to thelightpolarizing
dire-tion. This tehnique allows to hek the eÆieny of
theferrouiddopingwhenthesampleissubjetedtoa
magnetieldandalsotheeventualpreseneoflusters
ofmagnetigrains,indiatingaspuriousagglomeration
proess.
Anotheroptialtehniqueusedisthemeasurement
ofthetransmittaneasafuntionofthetimewith
sam-plessubjetedtomagnetields. Thesetuponsistsof
a polarized HeNe w laser (10 mW), two soures of
magneti elds, an analyzerand aphotodetetor
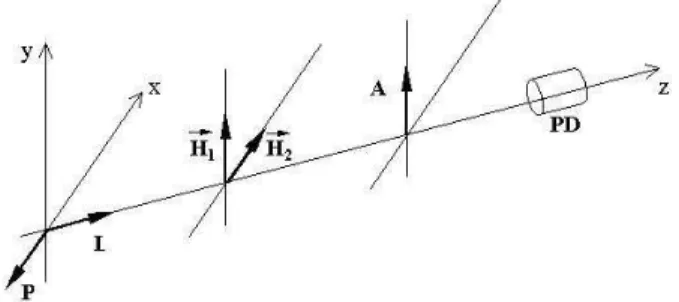
on-neted to aomputer. Figure 1showsa sketh ofthe
setup. The sample holders used are miroslides, with
thesamefeaturespresentedabove. Thelaserbeam
di-retion isparallel to the z axis(normalto thebiggest
atsurfaeofthemiroslide)anditswaistatthe
sam-ple'spositionin about1mm. Themagnetield His
asuperpositionoftwoindependentelds: astatiand
homogeneouseld (H
1
orientedalongthey axis)
gen-eratedby aneletromagnet(10 H
1
3000 G),and
another magneti eld H
2
(orientedalong the x axis)
isapulsedeld(strengthisbetween0and550G)
gen-eratedby twoHelmholtz oils. H
1
is orientedparallel
to thelongestaxisofthesampleholderand the
dire-tion ofthe analyzer. A pulse generator(square wave)
Figure 1. Sketh of the setup used to measure the
opti-altransmittaneof theferronematisamplessubjetedto
timedependentmagnetields.P,A,L,andPDarethe
po-larizer,analyzer,laserbeamdiretions,andphotodetetor,
respetively. x,y,andzarethelaboratoryframeaxes.
III Results
The lyotropi mixture doped with the water based
CuFe
2 O
4
ioniferrouiddidnotaeptwellthedoping.
At a onentration of C
o
= (71)10 13
grains/m 3
(C
o
C
m 10
8
grains/m 3
)itwasobserveda
preip-itation ofthegrains. Dueto anagglomerationproess
whih takes plae, the atual onentration of grains
in the solution is dierent from the initial one. The
nal solution presented a homogeneous brown olor.
With C C
m
thesample is transparent andno large
(mironsize)lustersareobserved. Thisfatindiates
that at the atual sample C
m
< C < C
o
. The value
ofC anbeestimated assumingthat,in theworst
sit-uation, 50% of the grains aggregate and, in this
situ-ation, C 310 13
grains/m 3
. Atthe preseneof a
magneti eld of 200 G it was veried the formation
of greatmagneti grainlusters withabout10 9
grains
eah (Fig. 2) . Comparing these results with those
ofthesamenemati sampledoped withawaterbased
surfated ferrouid (Fe
3 O
4
magneti grains of 10 nm
of mean diameter and magnetization at saturation of
100G),abigdiereneexists. It wasveriedthat the
surfatedferrouiddilutedeasilyinthelyotropiliquid
rystal. ContrarilytotheaseoftheCuFe
2 O
4
ioni
fer-rouid,grain lusterswerenotobserved. Theresponse
tomagnetieldisfastandeÆient. Thisresponsean
be evaluated in Figs. 3 (surfated ferronemati
with-outthe magneti eld)and 4(same sample 5seonds
after the appliation of a 200 G magneti eld). In
thepreseneofthemagnetield,sampletendsto
ori-entin aplanargeometry, with thediretorparallel to
the eld. The parallel lines observed in Fig. 4 are
wallsparallelto the appliedmagneti eld. Thesame
typeofdynami observation donewithCuFe
2 O
4 ioni
ferronemati sample indiated that the orientation of
thisferronemati(inthepreseneofthemagnetield)
om-the same degree of planar orientation. Figures 5and
6 show the CuFe
2 O
4
ioni ferronemati sample
with-out the eld and 10min after the appliation of the
magneti eld. Observing these gures, it is possible
to verifythat therearenotsigniantmodiationsin
theferronematitexture. Nevertheless,itwasverieda
smallhangeintheferronematitextureintheviinity
of the sample holder borders. This indiates that
al-thoughtheeÆieny ofCuFe
2 O
4
ferrouidisnotvery
goodtoorientlyotropiliquidrystals,itispossibleto
orientthem,butalongtimeisrequiredifomparedto
thesurfatedferronematiresults.
Figure 2. Ferronemati sample of CuFe
2 O
4
plaed in a
miroslide 400 mthik, subjeted to a magnetield of
200G,observedinapolarizedlightmirosope. The
mag-netigrainlusterisindiatedbythearrow.
Figure 3. Sample of surfated ferronemati in miroslide
400 m thik, observed in a polarized light mirosope,
Figure 4. Sample of surfated ferronemati in miroslide
400mthik, observed ina polarizedlightmirosope,in
thepreseneofamagnetield of200G.Thetexturewas
obtained5saftertheappliationofthemagnetield.
Figure 5. Sample of CuFe2O4 ioni ferronemati in
mi-roslide400mthik,observedinapolarizedlight
miro-sope,withouttheappliationofthemagnetield.
Figure 6. Sample of CuFe2O4 ioni ferronemati in
mi-roslide400mthik,observedinapolarizedlight
miro-sope,inthepreseneofamagnetieldof200G(alongthe
horizontaldiretion,intheplaneofthegure). Thetexture
We also studied the formation of NiFe
2 O
4 and
ZnFe
2 O
4
basedferronematisandthesamefeatures
ob-servedin theCuFe
2 O
4
ferronematiwereobtained. It
wasveriedthattheseferrouidsdonotdiluteverywell
inthelyotropiliquidrystal. Somegrains'aggregates
withtypialdimensionsofmironsan beobservedin
the texture under polarizing mirosope. The atual
onentration of grainsanalso beevaluated asdone
beforeandonehasC 310 13
grains/m 3
. The
over-all ferronematis present a brown homogeneousolor,
indiatingthat therearesomeindividualgrains inthe
liquidrystal matrix. Analyzing thebehaviorof these
ferronematissubjetedto low(oftheorder of200G)
magneti elds, we verify that signiant hanges do
notappearintheferronematistextureintimesofthe
orderofafewminutes. Alsointhisasethewallswere
formedin thetexturewhenthesample issubjetedto
themagnetield.
Figure7. Optial transmittaneas afuntionofthe time.
Sampleof ferronematiof ZnFe2O4 plaed inamiroslide
400mthik. Timeintervalofthepulset=100s.
The response time of the ZnFe
2 O
4
ferronemati
sample to largemagneti elds was quantitatively
in-vestigated. Due tothesimilaritiesbetweentheZnand
Ni based ferronematis behaviors in the presene of
smallmagnetieldsitisexpetedthattheresults
ob-tainedfortheZnbasedoneanbeextendedtotheNi
basedferronemati. Theexperimental setup used [17℄
is skethed in the Fig. 1. A onstant magneti eld
H
1
=2750Gandapulsedone(duringaninterval(t)
ofabout100s)H
2
=550Gareappliedtothesample.
Figure7showstheoptialtransmittaneasafuntion
of thetime. It is possible to seethat evenafter 100s
thetexturedidnotahieveastationaryonguration.
The transmittane presents an almost linear inrease
as a funtion of the time, indiating a weak
mehan-ial oupling of the diretor to the magneti grains.
As the mehanial oupling strongly depends on the
shapeanisotropyofthegrains(orevensmalllustersof
grains),itispossiblethatthisweakresponseofthe
ne-matimatrixto themagnetield(whihontrolsthe
quently,thegrainsorientationinthenematimatrix)is
duetothepreseneofalmostspherialgrains(orgrains
lusters). After 250 s the swithing o of the pulsed
magnetield,theoptialtransmittanedidnotreturn
toitsinitialvalue. Intheaseofsurfated
ferronemat-is,inthesameonditions,therelaxationtimeisabout
90s[17℄. Intheioniferronematisamplestudiedhere,
therelaxationtimealulatedis(191 3)s. Fortime
intervals t < 90s, during whih H
2
is present, the
transmittanedoessigniantlyhange,within our
ex-perimentalauray.
As aonlusion,due to this weakmehanial
ou-pling betweenthenemati diretorandthegrains,the
MFe
2 O
4 (M
2+
= Cu, Ni and Zn) based
ferronemat-is respond verybadly to small magneti elds. The
mehanial oupling does not depend on the size of
the magnetigrains but depends on thegrain'sshape
anisotropy[2℄. Intheeletronmirosopy
harateriza-tionofthegrains[5℄,theyappearroughlyspherial. A
small aggregation(dimers ortrimers)ouldfavourthe
mehanialouplingofthegrainswiththenemati
liq-uidrystal,but thisseemstobenottheasewith the
ioni ferrouids studied here. This results are
signif-iantly dierent from the one obtainedwith surfated
anditratedmaghemite(-Fe
2 O
3
)ioniferrouids[17℄.
Inthat ases,itwasveriedthat thetwotypesof
fer-rouid are eÆient to orient the nemati liquid
rys-tals. It was also veried that the surfated
ferrone-mati responds to the magneti eld faster than the
itratedferronemati. The dierenesobservedin the
surfated and ioni ferronematis ould indiate that
the amphiphili moleules of the surfated ferrouid
are importantin thestabilityof thelyotropi
ferrone-matis. Thebasiunits ofthelyotropiliquidrystals
aremielles(aggregatesofamphiphilimoleules).
Be-sidesthemielles, isolatedamphiphilimoleules exist
in the bulk of the nemati phase. As the grains in
surfatedferrouidsarealreadyoated withmoleules
having the same harateristis of the lyotropi
am-phiphili moleules,the magnetigrains ouldbe
ov-ered by them, inreasing the stability of the
ferrone-matisystem. With theionimagnetigrainsthis
sit-uation does not happen and ould explain why both
systemsbehavesdierentlyinthenematimatrix. Our
experiene with ferronematis (ioni and surfated) is
thattheyremainstableforseveralyears(atleastthree
yearsold samplespresentsthesamephysial-hemial
propertiesthanfreshones.Basedonthisfatwebelieve
that there are not hemial reations between grains
andmiellestakingplaein ferronematis.
Aknowledgments
Funda~aodeAmparo aPesquisadoEstado deS~ao
Paulo(Brazil),ConselhoNaionaldeDesenvolvimento
Referenes
[1℄ P.G.de Gennesand J.Prost, The Physisof Liquid
Crystals,2 nd
ed.(ClarendonPress,Oxford,1993)
[2℄ F.BrohardandP.G.deGennes,J.Physique31,691
(1970).
[3℄ R. E. Rosensweig, Ferrohydrodynamis (Cambridge
UniversityPress,Cambridge,1985).
[4℄ S.W.CharlesandJ.Popplewell,inFerromagneti
Ma-terial, editedbyE.P.Wohfarth(North-Holland
Pub-lishingCompany,Amsterdam,1980),Vol.2.
[5℄ F.A.Tourinho,J.Depeyrot,G.J.daSilva,andM.C.
L.Lara,Braz.J.Phys.28(4), 413(1998).
[6℄ R.Massart,USPatentNo.4329241(May1982).
[7℄ R.Massart,IEEETrans.Mag.MAG-17,1247(1981).
[8℄ S.H.ChenandN.M.Amer,Phys.Rev.Lett.51,2298
(1983).
[9℄ L.LiebertandA.Martinet,J.PhysiqueLett.40,L-363
(1979).
[10℄ L.LiebertandA.M.FigueiredoNeto,J.PhysiqueLett.
45,L-173(1884).
[11℄ A.M.FigueiredoNetoandM.M.F.Saba,Phys.Rev.
A34,3483(1986).
[12℄ A.M.FigueiredoNeto,Y.Galerne,A.M.Levelut,and
L. Liebert,inPhysisofComplex andSupermoleular
Fluids, editedby S.SafranandN. A.Clark, EXXON
MonographSeries(Wiley,NewYork,1987),p.347.
[13℄ A.M.FigueiredoNeto,Y.Galerne,A.MLevelut,and
L.Liebert,J.PhysiqueLett.46,L-499(1985).
[14℄ A.M.Figueiredo Neto,inPhase Transitionsin
Com-plexFluids,editedbyP.ToledanoandA.M.Figueiredo
Neto(WorldSienti,Singapore,1998),p.175.
[15℄ C.Y. Matuo, F. A. Tourinho, and A. M. Figueiredo
Neto,J.Magn.Magn.Mater.122,53(1993).
[16℄ J.C.BariandA. M.Figueiredo Neto,Phys.Rev.E
50,3860(1994).
[17℄ C.Y.MatuoandA.M.FigueiredoNeto,Phys.Rev.E
60,number2,1815(1999).
[18℄ E.A.Oliveira,A.M.FigueiredoNeto,andG.Durand,
Phys.Rev.A39,R825(1991).
[19℄ S. V. Burylov and Y. L. Raikher, Mol. Cryst. Liq.
Cryst.258,107(1995).
[20℄ Y.L.RaikherandV. I.Stepanov,J.Intel.Mat.Syst.
Str.7,550(1996).
[21℄ A.Yu.Zubarevand L. Yu.Iskakova,PhysiaA 229,
203(1996).
[22℄ F.A.Tourinho,P.C.Morais,M. H.Sousa, andL.G.
Maedo, Pro. 3rd Internat. Conf. on Intelligent
Ma-terials, edited by P. F.Gobin and J. Tatibouet (The
InternationalSoietyforOptialEngineering,
Belling-ham,Washington-USA),317(1996).
[23℄ A.M.FigueiredoNeto,L.Liebert,andY.Galerne,J.